| Annals of Burns and Fire Disasters - vol. X - n. 1 - March 1997
USE OF A BIOLOGICAL FILM AS A CULTURED EPIDERMAL
AUTOGRAFT SUPPORT IN THE TREATMENT OF BURNS: PRELIMINARY REPORT OF A NEW TECHNIQUE
Gueugniaud Fabreguette A. Oddou L, Petit P,
Collombel Damour
Lyons Burn Centre, Edouard Herriot Hospital,
Claude Bernard University, Lyons, France
Skin Substitute Laboratory, CNRS, 1341 URA, Lyons
SUMMARY.
The long-term outcome of the most critically burned patients is now in part linked to the
successful use of cultured human epidermal sheets. This technique however presents certain
disadvantages: a long delay before the sheets are available, the fragility and difficult
handling of the grafts, and the variability of the graft take rate, which is often poor,
particularly in some difficult areas (e.g. joints, back, neck), where the gauze used to
transfer the epidermal sheet impedes adherence of the sheet to the prepared wound. The aim
of this preliminary study is to assess a biological film for supporting keratinocyte
growth and transferring cultured epithelium to the wound without gauze transfer. The film
consists of 72% collagen, 20% chitosan and 8% glycosaminoglycans. The technique was used
in three patients following the surgical methods routinely used in our Centre, i.e. either
"combined" graft with conventional large mesh amograft covered with autologous
cultured sheets, or cultured epidermal autografts covering remaining allodermis after
early homograft. The culture time was shortened by several days because perfect confluence
of epidermal cells to achieve a multilayered epidermis was no longer necessary: cultured
grafts were available 14-16 days post-biopsy. The in vitro manipulation before grafting
allowed a saving of several hours by eliminating transfer on to a gauze. The areas thus
covered were the shoulder, flank, trunk, and forearm (range, 600 to 960 CM2). Clinically,
the seeded biological film was easy to handle and more adhesive to irregular wounds than
any classical dry or vaseline gauze. When the dressing was removed for graft verification,
the film disintegrated spontaneously, without detrimental effects to initial take. The
success rate ranged from 85 to 98% after the first grafting procedure. In conclusion, the
film seems to be useful as it accelerates keratinocyte cutture. It permits good
co-ordination between the biological and the medical teams, and it therefore appears to be
a valid technical alternative to conventional cultured epidermis, especially in mobile
zones.
Introduction
The prognosis for the most critically
burned patients has considerably improved in the last few years thanks to the progress
achieved in resuscitation techniques. Fluid expansion was the first step in this
progress.' The better understanding of the initial haemodynamic disorders of patients with
major thermal burns has improved vital prognosis The full recovery of these
critically burned patients who are today successfully resuscitated cannot be achieved
without the use of skin substitutes.
First described twenty years ago,' cultured epithelium allows complete coverage of the
skin, which is impossible to achieve with traditional grafts because of the limited donor
sites.' The main hope for the future regarding the treatment of very severely burned
patients lies in the success of cultured epithelium, the production of which is now fully
under the biologists' control. As regards burn coverage, Gallico was one of the first
surgeons to use epidermal sheets obtained by Rheinwald and Green's rnethod.` Within three
weeks, this technique enables medical teams to have at their disposal a considerable
quantity of cultured epidermal autografts (CEA) which can be grafted on to an area
previously prepared by surgical excision of burned tissues. The most effective method of
protecting the wound bed, pending grafting with CEA, is considered to be the use of
engrafted allodermis via early homografts.
However, the clinical success of cultured epithelium remains variable: the literature
reports an average success rate ranging from 30 to 70%. Several factors affect the success
of CEA take, among which we may schematically distinguish clinical factors related to the
patient and biological factors related to the culture technique and the transfer of the
sheets.
With regard to clinical factors, the quality of the patient's receiving site is of prime
importance for the success of CEA: the preliminary surgical excision must prepare a clean
and well-vascularized wound to receive CEA. Today, after excision, the temporary
application of homografts (when available) allows a better preparation of these areas
pending permanent coverage. This preparation is however usually disturbed during the first
three weeks by deterioration of the patient's general condition. The success of CEA is
also compromised by initial haemodynamic disturbances, decreased immunity, denutrition
and, above all, problems of local or general infection.
Thus, as far as clinical factors are concerned, improvement of the technique of cultured
epithelium requires a reduction in the time taken to create the sheets. This would prevent
serious sepsis, which is often responsible for premature non-take of the temporary
homograft or permanent autograft.
With regard to biological factors, the culture technique of the epidermal cells and the
phase of the transfer of the sheets are both necessary to achieve a successful graft.
Today, in addition to confluence of the keratinocytes, it is necessary to achieve good
cohesion between the cells and to dispose of a strong sheet that can be be transferred on
dry petrolatum gauze: it thus takes about 21 days to obtain CEA. This period could be
reduced if confluence ceased to be a necessity and if cell proliferation could continue
after transfer on to the receiving site itself.
An ideal support would have other requirements and could be defined according to certain
biological and clinical characteristics. Biologically, the culture could be produced
directly on the support, where the cells could adhere and proliferate. This support would
be biocompatible and easy to handle, and it would allow a shorter transfer time.
Clinically, the support would have to be: a) flexible, to allow total contact between CEA
and the receiving site; b) thin and permeable, to prevent any risk of complete occlusion
of the wound and to facilitate the escape of exudates from the underlying tissues; and c)
biodegradable, for easy removal of the biornaterial when the graft is checked.
Following the initial collagen sponge model proposed by Yannas, the first biological films
to be developed were collagen films, which are now successfully used for burn coverage.
Subsequently, other films were used, composed of materials such as hyaluronane, chitin,
fibronectin, and fibrin molecules. Fibrin offers a number of possibilities: the cells can
be seeded on a fibrin film or incorporated into the fibrin to be sprayed on to the wound.
We present here a film made of collagen, glycosamino-glycans (GAG), and chitosan. The aim
of our study is to assess this biological filtr as a support for the development of
keratinocytes cultured in vitro and for the transfer of CEA on to the receiving
site, without the use of any artificial support. Compatibly with the condition of the
patient, the surgical techniques adopted in our Centre (after early excision down to the
adipose tissue) consist either of homografts when available, followed two weeks later by
CEA covering any remaining allodermis, or - as soon as possible - "combined"
grafts, with a large mesh autograft covered with cultured epidermal sheets: for the
clinical reports, the same techniques are tested in this preliminary study.
Material and method
Material: the seededfilm
The cells
The keratinocytes were isolated from the patient's healthy skin biopsy by
trypsinization overnight at 4 'C. The keratinocyte suspension was seeded on a feeder layer
made of human irradiated foreskin fibroblasts, in a medium containing Dulbecco's modified
Eagle's medium (DMEM) and HAM F-12 (SIGMA Laboratories), supplemented with 10% of foetal
calf serum (SIGMA Laboratories) and 0.4 mg/ml hydrocortisone, 5 mg/ml insulin, epidermal
growth factor (EGF), 5 mg/ml transferrin, 2 x 10-1 mol/ml tri-iodothyronine, 10-11 mol/ml
choleratoxin, and 8 x 10` mol/ml adenine (SIGMA Laboratories). The cultures were incubated
at 37 'C in a humidified atmosphere containing 5% C02, and the medium was changed three
times weekly. After three days, 10 ng/ml EGF was added to the medium. When the primary
culture was nearly confluent, the keratinocytes were resuspended by trypsinization for
secondary culture or film seeding.
The components of thefilm
Our film was made of collagen, glycosaminoglycans (Chondroitin-2 and 4-Sulphate) (GAG) and
chitosan, all purchased from SADUC (Lyons, France). The biosafety of the components was
checked in conformity with CEE recommendation number 111/3298/91 (Guidelines for
minimizing the risk of transmissible agents causing spongiform encephalopathy via medical
products).
Method
Preparation of thefilm
As we found previouSly,2' the optimal composition for the preparation of the film is 72%
collagen, 20% chitosan and 8% GAG. This is prepared in large sheets and left to dry. The
films are sterilized by gamma irradiation (25 kG).
Culture conditions
Before use, the film is washed twice with sterile phosphate buffered saline, then
equilibrated with DMEM. At the optimal pH of 7 the cells are seeded on the film in
rectangular metal frames at a density of 101 cells per CM2 . The frames are removed three
hours after seeding, when the cells are attached to the film. This cell density (used in
the three cases described) is near subconfluence and allows grafting of the film one day
after seeding. A lower density can be used if the graft is expected later, allowing
modulation.
Controls
Our film is not transparent enough to allow a lightmicroscope evaluation of the cells on
the film. We therefore looked for a macroscopic method that would permit appreciation of
the cell density we required. We used a colorimetric method known as the MTT test which is
normally employed to evaluate cell viability: a yellow tetrazolium salt is reduced by the
viable cells to an insoluble blue formazan product. Before the graft, a small portion of
the film was taken and incubated in NITT solution. After 30 minutes' incubation, direct
observation of the blue area allowed evaluation of viability and confluence.
Case reports
The seeded film was tested for partial
skin covering in three patients, with the approval of the Lyons University Hospital Ethics
Committee.
In our Centre, virus-free donor skin is applied, if available, after early scar excision
in order to prepare wounds for CEA. However, so long as donor sites remain valid, we use
CEA to cover conventional large mesh autografts, especially in difficult areas, e.g.
joints and the back.
These techniques were applied for seeded film in the following three case reports (Table
1).
Case 1
An I I -year-old boy, a fire casualty, was admitted to our Burn Centre with burns of
80% T13SA (60% full skinthickness and 20% deep partial). His state of health required over
two months' stay in the intensive care unit, with artificial ventilation for 45 days. A
skin biopsy for keratinocyte culture was harvested on day 4 post-burn. During the first
two weeks, local treatment consisted of complete surgical excision of the burned areas in
three weekly surgical sessions, with initial covering by collagen dressing,` because no
homograft was available. The excised wounds rapidly appeared favourable for grafting and a
first graft session was planned at the end of the second week of treatment to cover the
superior limbs and the left hemitrunk. At this point, traditional CEA was not yet
available as the keratinocytes were not yet confluent, and we therefore decided to seed
some biological films two days before the graft session. As the donor sites were extremely
limited, "combined" grafts with mesh 6:1 autograft covered with eight seeded
films (12 x 10 cm) were layered on to the side of the left hemitrunk 14 days after biopsy.
When the graft was checked on day 8, the result of this "combined" autograft on
the left half of the trunk was a 98% success; also, the large openings of the 6:1 mesh
under the films had completely disappeared in the same short period. The successive grafts
were produced using conventional cultured keratinocytes available on dry gauze. The
patient was discharged alive on day 98 postburn.
| Patient |
Age
(yr) |
TBSA
(%) |
Post-biopsy
day of
SF graft |
SF coverage cm² |
SF graft
technique |
Grafted
area |
Average
initial take
(%) |
Average
final take
(%) |
| 1 |
11 |
80 |
14 |
960 |
Autograft
covered
by SF |
Left hemi-
trunk |
98 |
100 |
| 2 |
40 |
45 |
16 |
120 480 |
Autograft
covered
by SF |
Left shoulder Back lellside |
90 80 |
96 90 |
| 3 |
13 |
85 |
15 |
120 |
SF grafted
on allodermis |
Left forearm
and elbow |
85 |
85 |
|
Table I
- Patient data for seeded film (SF) testing |
|
Case 2
A 40-year-old man was burned by
blazing gas when attempting to take his life, suffering 35% TBSA full skin-thickness burns
and 10% deep partial burns. The areas concerned (only partially) were the head, the front
and back of the trunk, and the upper limbs. The patient also presented an important
primary respiratory lesion, and his initial state necessitated artificial respiration
after tracheotomy for three weeks. The haemodynamic condition remained unstable, requiring
pulmonary artery monitoring and the use of catecholamines. Prolonged circulatory shock
prevented surgical debridement during the first week, and initial local wound therapy
consisted of silver sulphadiazine dressings. Owing to the delay in excision, and
considering the size and depth of the wounds, a skin biopsy was taken on day 7
post-admission.During the second week of treatment, three excisiongraft sessions were
performed. From the surgical point of view, the burns quickly reached a satisfactory
state, and the quality of the autografts permitted excellent coverage before the end of
the third week. However, one last surgical session proved necessary in order to complete
the grafts as some areas had not completely healed: at that moment, the confluence of the
keratinocytes was not perfect, and a conventional sheet was not possible: some films (600
cm') with autologous keratinocytes were made on day 22 post-burn (corresponding to day 15
post-biopsy) and used the following day both on welldefined spots to complete the
traditional graft (left flank) and on the left shoulder to cover a dermo-epidermal graft
in wide mesh (mesh M) according to the "combined" technique.
The initial results obtained with this technique on the patient were satisfactory, with
healing in approximately 85% of the zones covered (90% in the shoulder). We present here
the photographs of the graft performed on the left shoulder, with a 3:1 mesh
dermo-epidermal graft, with half the external area covered by the seeded film (the
"combined" technique). The internal part (near the neck) was grafted only with
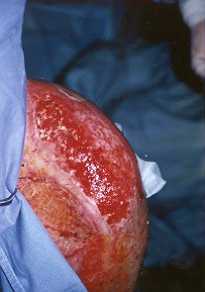
autografts, and can thus be regarded as a control area. Fig. ]a shows the aspect of
the wound bed before grafting. Although the zone is irregular and not flat, it is possible
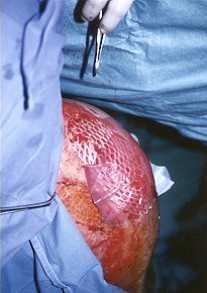
to observe perfect adhesion to the film to the wound (Fig. lb). As the zone was in
a mobile joint, a dry gauze was spread over the graft to keep it adherent to the wound.
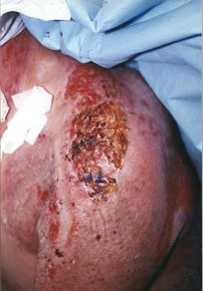
The result of this graft on day 6 is presented in Fig. ]c and on day 10 in Fig.
M. Fig. ]c shows the dry brown aspect of the film which partially hides the underlying
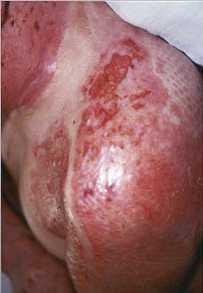
dermo-epidermal graft. Fig. ]d highlights the excellent rate of take, with complete
healing of the mesh holes under the seeded film, unlike the control zones (Fig. M). There
was partial take of the autograft on the film-free area. The patient was discharged on day
45.
Case 3
A 13-year-old child suffered burns in a
brushwood fire involving 85% T13SA (75% full skin-thickness). On day 2 post-burn a culture
biopsy was harvested from the unhurried scalp, during the first escharectomy session.
Further excisions down to the adipose tissue were performed in the anterior side of the
trunk on day 5 and the upper limbs on day 8, with coverage by fresh meshed cadaver skin.
When the allogfaft was checked seven days later, the clinical result was particularly good
in the left forearm. At this early stage of treatment the patient was free of local or
general infection. We therefore decided to cover the forearm with autologous
keratinocytes, and six biological films were immediately seeded at a density of 101 cells
per cral. On day 15 post-biopsy, seeded films were applied on the left forearm and elbow
after surgical alloepidermis removal. At the first dressing change seven days later, the
primary take rate was only 85% because the sides of the graft were destroyed. However, one
week later, the appearance of the healed skin was excellent. The patient was discharged on
day 82 after 65 days in the intensive care unit.
|
|
Fig.
la - Aspect of wound after debridement of left shoulder. |
Fig.
1b - Graft performed with 3:1 meshed autograft. Only the external area is covered
with film seeded with autologous keratinocytes.
The internal part is a control area. |
|
Discussion
The successful use of cultured
epithelium depends on both biological and clinical factors. The biological aspect is now
perfectly codified. However, in vitro development can still be improved, and two
points seem to be of particular importance.
First of all, clinicians generally find that it takes too long to produce CEA. A reduction
of a few days would mean great progress, especially when homografts are unavailable. The
use of the film presents numerous advantages: it obviates the need of a transfer step on
petrolatum gauze, and the time taken for culture is shortened as perfect confluence of the
cells is no longer needed to make the graft possible, and cell differentiation can also be
avoided. The time taken to produce an epidermal culture before its clinical application
can thus be shortened by four to seven days. In Case 1, the early
"combined" graft permitted a spectacular result on a very large mesh. In Case
2, we cl obtained good healing of the joint, but only under the film. Joints
are normally considered unfavourable areas, unsuitable for conventional grafts. In Case
3, the early cultured cells autografted on the remaining allodermis of the forearm
were easier to graft and more efficient than the later traditional sheet autografts
performed during a persistent and resistant sepsis syndrome (due to Pseudomonas) that
occurred one week after the seeded film graft.
Secondly, it is difficult to predict the general state of health of burn patients:
sometimes a graft session has to be advanced a few days. As the culture time is shortened,
the cells are not sufficiently confluent to allow the production of conventional sheets.
They can therefore be trypsinized and seeded for one night on the film. On the other hand,
in the event of a contaminated wound or severe deterioration of the patient's general
conditions, a graft may have to be postponed. CEA cannot however be used too long after
confluence, because the keratinocytes lose their proliferative power. To solve this
problem, the cultures can be trypsinized before confluence and their differentiation. They
can be seeded on the film, with a density relative to the delay of the graft. From the end
of week 2 post-burn, seeded film grafts can be planned even one day before surgery.
It is also important that the biological film preserves the epidermal cells in an active
proliferating state. Here we support Kaiser et al., who proposed the application to burn
wounds of non- confluent cultured autologous keratinocytes in a suspension of fibrin glue.
|
 |
| Fig.
1c - Result of graft on day 6: brown aspect of dried film partially concealing
wound healing. |
Fig.
ld - Result of graft on day 10: complete healing under film. Control area
presents incomplete healing. |
|
With regard to biological requirements,
the film is ideally biocompatible. The MTT test, which is basically a cytocompatibility
test, confirmed the absence of toxicity, and the fact that the cells proliferated proves
that our film is an excellent support for in vitro cell culture. The film is easy to
handle in vitro, and the adherence of cultured keratinocytes is excellent. With regard to
clinical conditions, the film is easy to use as a support. Its flexibility is very useful
because it adheres perfectly to the receiving site, achieving total contact with the
seeded film (e.g. Case 2) and partial contact with gauze in "difficult" sites.
Its permeability also allows exudates to pass through from the receiving site, and this
reduces soaking. The film completely disintegrates within five to eight days - a period
corresponding to the take of epidermal grafts. It is thus possible to avoid removal of the
support, which is always particularly critical and often deleterious (due to adhesion to
the wourid and/or haernorrhagic removal).
Conclusion
The preliminary results of the use of
this biological film as a support to grow keratinocytes in subculture and as a transfer
support look very promising as regards the ranges of application that have been tested,
especially in mobile zones and joints. This new approach is promising both biologically
and clinically, and justifies further research with a view to improving its use as a
technical alternative to cultured autologous epithelium in the care of the most critically
burned patients.
RESUME. La survie
des brûlés les plus sévères passe par l'utilisation des feuillets d'épiderme humain
cultivé. Les principaux inconvénients de cette technique sont les délais avant
l'obtention des feuillets, leur fragilité ainsi que leur faible maniabilité. De plus,
les pourcentages de prise de greffe sont variables et parfois faibles, particulièrement
pour les zones difficiles, comme les articulations, le dos et le cou, où les gazes de
transfert des feuillets ne sont pas suffisamment adhérentes au lit de la plaie. Cette
étude préliminaire décrit l'utilisation d'un film biologique permettant la culture de
kératinocytes et le transfert des cellules directement sur la plaie sans gaze de
transfert. Le film (composé de 72% de collagène, 20% de chitosane et 8% de
glycosaminoglycanes) a été utilisé chez trois patients qui avaient été traités par
les méthodes chirurgicales habituellement utilisées dans notre Centre: soit la méthode
"combinée" avec des feuillets épidermiques cultivés recouvrant une autogreffe
dermo-épidermique largement meshée, soit les feuillets d'épiderme cultivé recouvrant
une allogreffe après excision de l'épiderme. Les temps de culture ont pu être
raccourcis de quelques jours puisque la parfaite confluence cellulaire ainsi que
l'obtention d'un épiderme pluristratifié ne sont plus nécessaires: ainsi les cellules
épidermiques ont pu être greffées entre le 14e et le 16e jour après la biopsie. Les
manipulations nécessaires au transfert du feuillet sur la gaze, qui prenaient quelques
heures, ne sont également plus nécessaires. Les zones ainsi recouvertes sont: une
épaule, des flancs, un dos et un avant-bras, avec des surfaces allant de 600 à 960 cm'.
D'un point de vue clinique, le film est facile à manipuler et beaucoup plus adhérent à
la plaie que les pansements classiques ou vaselinés. Quand le pansement est enlevé lors
de la vérification de la greffe, le film se désintègre spontanément sans effet
délétère pour la prise de greffe, qui etait de 85 à 98% en moyenne après la première
greffe. En conclusion, le film en accélerant la croissance des kératinocytes permet une
bonne coordination entre l'équipe médicale et les biologistes. Il semble être une
alternative technique possible à l'utilisation des feuillets épidermiques, plus
spécialement pour les zones mobiles.
BIBLIOGRAPHY
- Baxter C.: Fluid volume and electrolyte changes in the
early post burn period. Clin. Plast. Surg., 1: 693-709, 1974.
- Dries D.J., Waxman K.: Adequate resuscitation of burn
patients may not be measured by urine output and vital signs. Crit. Care Med., 19: 327-9,
1991
- Bernard F., Gueugniaud P.Y., Bertin-Maghit M. et al.:
Prognostic significance of early cardiac index measurement in severely burned patients.
Burns, 20: 529-31, 1994.
- Gueugmaud PY., Vilasco B., Pham E., Hirschauer C., Bouchard
C., Fabreguette A., Petit R: Brûlés graves: état hémodynamique, transport et
consommation d'oxygène, cytokines plasmatiques. Ann. Fr. Anesth. R6anim., 15: 27-35, 1996
- Rheinwald J., Green H.: Serial cultivation of strains of
human epidermal keratinocytes: the formation of keratinizing colonies from single cells.
Cell, 6: 331-44, 1975.
- Gallico G., O'Connor N., Compton C. et al.: Permanent
coverage of large burn wounds with autologous cultured human epithelium. N. 0. EDg. J.
Med., 311: 448-51, 1984.
- Green H., Kehinde 0., Thomas J.: Growth of cultured human
epidermal cells into multiple epithelia suitable for grafting. Cell Biology, 11: 5665-8,
1979.
- Green H.: Cultured cells for the treatment of disease. Sci.
Am., 11: 96-102, 1991.
- Hickerson W.L., Compton C., Fletchall S., Smith L.R.:
Cultured epidermal autografts and allodermis combination for permanent burn wound
coverage. Burns, 20: S52-6, 1994.
- Damour 0., Bertin-Maghit M_ Oddou L. et al.: Cultured
autologous epidermis combined with large meshed autografts for burn treatment of children.
Ninth ISBI Congress, Paris, July 1994.
- Bolivar Flores J., Poumian E., Marsch Moreno M. et al.: Use
of cuttured epidermal keratinocytes for allografting burns and conditions for temporary
banking of cultured allografts. Burns, 16: 3-8, 1990.
- Cuono C., Langdon R., McGuire J.: Use of cultured
allografts and dermal allografts as skin replacement after burn injury. Lancet, 1: 1123-4,
1986
- Eldad A., Burt A., Clarke J.A.: Cultured epithelium as skin
substitute. Burns, 13: 173-80, 1987.
- Foyatier J.L., Faure M., Hezez G. et al.: Applications
cliniques des greffes d'épidermes cultivés chez le brûlé: à propos de 16
observations. Ann. Chir. Plast. Esthet., 35: 39-46, 1990.
- Still J.M., Orlet H.L., Law E.J.: Use of cultured epidermal
auto grafts in the treatment of large burns. Burns, 20: 539-43, 1994.
- Carsin H., Rives J.M., Le Reveille R., Le Bever H., Ainaud
P.: Les autogreffes d'épithélium cultivé dans la thérapeutique du grand brûlé.
Concours Médical, 118: 983-8, 1996.
- Tompkins G., Burke L: Alternative wound coverings. In:
"Total Burn Care", 165-72, Herndon (Ed.). W.B. Saunders, Philadelphia, 1996.
- Gueugmaud P.Y., Bertin-Maghit M. Burn therapy. Current
Opinion in Anesthesiology, 8: 187-92, 1995.
- Yarmas L, Burke J.: Design of an artificial skin: 1. Basic
design principles. J. Biomed. Mater. Res., 14: 65-81, 1980.
- Moryhwas M., Stevenson 1, Marcelo C., Thornton L, Smith D.:
In vitro and in vivo testing of a collagen sheet to support keratinocyte growth for use
as burn wound covering. J. Trauma, 29: 1163-7, 1989.
- Koide M., Osaki K., Konishi J. et al.: Anew type
ofbiornaterial for artificial skin: dehydrothermally crosslinked composites of fibrillar
and denaturated collagen. J. Biomed. Mater. Res., 27: 79-87, 1993.
- Andreassi L., Casim L., Trabucchi E. et al.: Human
keratinocytes cultured on membrane composed of benzyl ester of hyaluronic acid suitable
for grafting. Wounds, 3: 116-26, 1990.
- Kishimoto S., Tamaki K.: Irrammohistochemical and
histochemical observations in the process of wound healing in guinea pig skin under
chitine membrane dressing. Acta Dennatol. Kyoto, 82: 471-9, 1987.
- Brown R., Gordon W., Ejim 0.: Preparation of orientated
fibrous mats from fibronectin: composition and stability. Biomaterials, 15: 457-64, 1994.
- Hafemarm B., Hettich R., Ensslen S. et al.: Treatment of
skin defects using suspensions of in vitro cultured keratinocytes. Burns, 20: 168
72, 1994.
- Kaiser H.W., Stark G.B., Kopp L, Balcerkiewiez A., Spilker
G., Kreysel H.W.: Cultured autologous keratinocytes in fibrin glue suspension exclusively
and combined with STS-allograft (preliminary clinical and histological report of a new
technique). Burns, 20: 23-9, 1994.
- Hunyadi J_ Farkas B., Bertenyi C_ Olah J_ Dobozy A.:
Keratinocyte grafting: a new means of transplantation for full thickness wound. J.
Dermatol. Surg. Oncol., 75-8: 1988.
- Collombel C., Damour 0., Gagnieu C. et al.: Peau
artificielle et son procédé de fabrication. Brevet français NN 87-087752, 1987, et
Brevet européen N88-420194, 1988.
- Mossman 1: Rapid colorimetric assay for cellular growth and
survival: application to proliferation and cytotoxicity assay. J. Immunol. Methods, 65:
55-63, 1983.
- Hansen M., Nielen S., Berg K.: Re-examination and further
development of a precise and rapid dye method for measuring cell growth and cell kill. J.
Immunol. Methods, 116: 203-10, 1989.
- Oamour 0., Gueugniaud RY, Bertin-Maghit XL, Rousselle P_
Berthod E, Collombel C.: A dermal substrate made of collagenGAG-chitosan for deep burn
coverage: first clinical uses. Clin. Mat., 15: 273-6, 1994.
This paper was
received on 2 September 1996.
Address correspondence to: Dr P.Y. Gueugmaud,
Service des Brûlés, SAR VIL Hôpital Edouard Herriot,
5 Place d'Arsonval, 69437 Lyon Cedex 03, France.
Acknowledgements. We thank
Annic Lepavec for her
technical assistance and Helen Drew for her assistance in the
perusal of the paper. This work was partially supported
by DRET N° 90226, INSERM N° 886901, CNRS, Hospices
Civils de Lyon and Fondation Rhône Alpes Futur. |
|



