| Annals of Burns and Fire Disasters - vol, X - n. 1 - March 1997
HISTOLOGICAL
AND IMMUNOHISTOCHEMICAL EVALUATION OF HUMAN CULTURED EPIDERMAL CELLS
Garcia Fernàndez E.,(1) Bejar J.M.(2) Maruri
M.,(1) Garcia Masdevall M.D.,(1) Camarero C.(3) Gabilondo FJ .(2)
(1) Department of Immunology, Hospital cle Cruces,
Baracaldo, Vizcaya, Spain
(2) Department of Plastic Surgery and Burns, Hospital cle Cruces
(3) Department of Pathological Anatomy, Hospital cle Cruces (Basque Health Service)
SUMMARY. The cutaneous
coverage of large and deep skin defects secondary to burn wounds or certain diseases is a
complex surgical problem. Normal human keratinocytes can be serially cultured in vitro,
and in appropriate culture conditions give rise to a stratified squamous epithelium.
Although no stratum corneurn is developed in vitro, these cultures enable us to
study early events in the development, proliferation, and differentiation of human
keratinocytes. For a better understanding of these findings, a study was made of cell
viability and the histological (morphological analysis) and immunohistochemical picture in
epidermal sheets, tested at different culture times, as compared with normal human skin
from foctuses, newborns, and adults. It was observed that cultures a few days old are
similar to foetus and newbom skins as regards immunohistochemical expressivity (but not
the number of cell layers). Adult skin is more similar to secondary cultures as regards
its superficial differentiation, slight positivity to involucrin, and larger number of
cell layers, although the cultures never present such a high number of cell layers as
adult skin.
Introduction
The cutaneous cover of large and deep
skin defects secondary to burn wounds and certain diseases presents a complex surgical
problem. Normal human keratinocytes can be serially cultured in vitro,' and in
appropriate culture conditions give rise to a stratified squamous epithelium.' Cultured
human epithelium possesses a site-specific differentiation programme which is expressed
after grafting, independently of the receiving body site. The growth and differentiation
of keratinocytes and the development of a new dermo-epidermal junction are influenced by
culture techniques and epithelial sheet handling. For a better understanding of the
development, proliferation, and differentiation of in vitro cultured human
epidermal cells, a study was made of cell viability and the histological (morphologic
analysis) and immunohistochemical picture in epidermal sheets, tested at different culture
times.
Materials and methods
Culture of human epidermal cells
The skin samples, biopsies, and skin grafts were placed in a culture medium and
transferred to the laboratory for cultivation. They were washed twice in DMEM (Gibco BRL
Co.) with 10% bovine foetal serum (Gibco BRL Co.) and antibiotics (penicillin 100 u/ml,
streptomycin 0.1 mg/ml and Fungizone 0.25 ug/ml) (Sigma Chemical Co.). After removal of as
much subcu-aneous tissue and dermis as possible, the tissue was cut into smaller
fragments, which were digested overnight with 0.17% trypsin solution (Seromed. Biochrom
KG). This was followed by treatment with trypsin-EDTA (25% - 0.02 mM) solution (Seromed.
Biochrorn KG) to ensure dissociation into single cells. The single cells were then washed,
and after centrifugation and suspension in complete medium the viability of epidermal
cells was determined by trypan blue (Sigma Chemical Co.) dye exclusion.
Plastic tissue-culture 75 cm' flasks (Costar Co.) already containing 2.5 x 10' lethally
irradiated murine 3T3 fibroblasts (European Collection of Animal Cell Cultures) were
inoculated with over 3 x 10' epithelial cells/cml. The cultures were fed with a 3:1
mixture of the Dulbecco-Vogt modification of Eagle's medium and Ham's F-12 medium (Gibco
BRL Co.). Supplements were as follows: foetal calf serum 10% (Gibco BRL Co.), adenine
24,ug/ml (Sigma Chemical Co.), epiden-nal growth factor 10 ng/ml (Sigma Chemical Co.),
hydrocortisone 0.5 ng/ml (Sigma Chemical Co.), insulin 5 i)g/i-nl (Gibco BRL Co.), cholera
toxin 6 ng/ml (Gibco BRL Co.), transferrin 10 i)g/nal (Gibco BRL Co.) and
3,3',5-triodothyronine 1.3 ng/ml (Sigma Chemical Co.). The cultures were incubated at 37
'C in a humid 95% C02 environment (Selecta Co.); the medium was changed every third day.
To prepare secondary cultures, subconfluent primary cultures were trypsinized and the
cells were transferred to flasks of the same size containing irradiated 3T3 cells. These
cultures were maintained in the same way as the primary cultures.
Cell viability study and cell number
For the cell viability study we used
32 epidermal sheets tested at different culture times. The cells were detached with
trypsin solution from 75 CM2 plastic flasks and cell viability was determined by
trypan blue dye exclusion.
Histological techniques
We studied three primary cultures
after 15 days of evolution, three primary cultures after 30 days, and four secondary
cultures after at least 30 days. As control samples we studied foetus (29 weeks'
gestation), newborn, and adult skin obtained from the Service of Pathological Anatomy
(Hospital de Cruces).
For the morphological analysis the medium was decanted off and the cultures were washed
three times with sterile PBS (Gibco BRL Co.). A solution of dispase 11 (Boehringer
Mannheim) was added to cover the entire cell sheet for incubation at 37 'C for 30 min. The
detached epidermal sheets were placed in sterile petri dishes and the dispase was removed
by washing carefully three times with PBS.
The sheets were fixed by the addition of 10% buffered formalin, embedded in paraffin,
sectioned and stained with haematoxylin-eosin for optic microscopic examination. In these
sections, the number of cell layers and the basal, intermediate and superficial
distribution were analysed.
Immunohistochemistry
The sections of paraffin-embedded were
cleared in xylene, rehydrated in a graded series of alcohol rinses, and reacted with
monoclonal antibodies: AEI (acidic cytokera tins of low molecular weight which mark the
epidermal basal layers) (BioGenex Laboratories), AE3 (basic cytoke ratins of the
suprabasal and occasionally basal layer') (BioGenex Laboratories), 5D3 cytokeratins 8, 18,
and 19 (marker indicating columnar cell differentiation and glan dula,r epithelium)
(BioGenex Laboratories), and involucrin(protein component of the cross-linked envelope
synthesized by maturing cells of human stratified squamous epithelium, reflecting no ,
rmal suprabasal differentiation9) (Biomedical Technologies Inc.). The visualization system
used was LSAB, marked with peroxidase and development was with AEC (AminEthyl-Carbazol).
Results
The results at different culture times by
optic microscopy suggest that the greater the number of days the cells stay in culture,
the higher the average number of cells after flask trypsinization. Cell viability on
culture days 5 and 8 is very low (57.1% and 66.4% respectively) but on successive days
viability improved in value to 80-100% in samples (Table 1).
Sample
(days of culture) |
N° samples
|
N° cells
(average) |
Viability (%)
(average) |
| 5 |
1 |
400.000 |
57.1 |
| 8 |
1 |
1.050.000 |
66.4 |
| 11 |
2 |
2.315.000 |
91.9 |
| 13 |
4 |
3.612.500 |
82.5 |
| 14 |
3 |
3.292.333 |
85.8 |
| 15 |
2 |
5.600.000 |
100.0 |
| 16 |
2 |
6.960.000 |
100.0 |
| 20 |
1 |
5.565.000 |
80.5 |
| 30 or more |
16 |
11.858.338 |
89.5 |
|
Table I -
Average number of cells after 75 cr& flask trypsinization and cell viability at
different culture times |
|
During the early days of
growth in culture, the keratinocytes proliferated as small colonies which coalesced into
confluent sheets of cells within 15 days. The cultures could then be lifted from the
plastic as an intact sheet. Histological sectioning of a 15-day-old epidermal cell sheet
revealed that the cultures had a thickness of between one and three cell layers. The
primary cultures of a 30-day-old sheet were slightly more stratified (two to four cell
layers), while secondary cultures at least 30 days old attained thicknesses ranging
between four and six cell layers.
During the latter stage of growth, secondary cultures were composed of small rounded
basal cells attached to the plastic substrate and suprabasal layers consisting of
enlarged, irregularly shaped vacuolated cells resemblimg the stratum germinativurn and
spinosum. Although no stratum corneum developed in these cultures the cells retained the
capacity, once grafted, to differentiate terminally.
Optic microscopy showed that epithelial thickness slightly increased in relation to the
culture time and the passage number (primary and secondary cultures). The basal layer (Fig.
1) was also more evident and organized in relation to the culture time and the passage
number, i.e., the epithelium appeared to have more organization. The expression of basal
and immediately suprabasal keratins was however highest in 15-day-old primary cultures and
decreased in 30-day-old primary cultures and in secondary cultures.
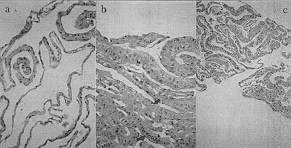 |
Fig. 1 - Histological sectoring:
a) 15-day-old primary culture
b) 30-day-old primary culture
c) secondary culture at least 30 days oldNote the basal layer and
the keratholyne granules in intermediate layer cells and the most superficial cells |
|
The epithelial differentiation which
appeared in the expressivity of intermediate layers was highest in 30-dayold primary
cultures and decreased in secondary cultures.
|
| Case |
Type/time
of culture |
General
description |
AEI |
AE3 |
5D3 |
Involucrin |
| 1 |
Primary cultures
15 days old |
2-3 cell layers.
Basal layer+ |
++ |
++ |
Not evident |
Not evident |
| 3 |
2-3 cell layers.
Basal layer+ |
++ |
++ |
Not evident |
Not evident |
| 5 |
2-3 cell layers and
some zones 4.
Basal layer+ |
++ |
++ |
- |
- |
| 2 |
2-3 cell layers and
some zones 4.
Basal layer+ |
+++ |
+++ |
Not evident |
Not evident |
| 4 |
Primary cultures
30 days old |
2-3 cell layers.
Basal layer++ |
++ |
+++ |
+ |
Not evident |
| 6 |
2-3 cell layers and
some zones 4.
Basal layer+ |
+ |
++ |
Not evident |
- |
| 7 |
3-4cell layers.
Basal layer+ |
+ |
+ |
- |
Weak+ |
| 8 |
3-4 cell layers and
some zones 5.
Basal layer+ |
+ |
+ |
- |
Weak+ |
| 9 |
Secondary
cultures
at least 30 days old |
3-4 cell layers and
some zones 5.
Basal layer+++ |
Weak+ |
+ |
- |
Weak+ |
| 10 |
3-4 cell layers and
some zones 5.
Basal layer+++ |
Weak+ |
+ |
- |
Weak+ |
|
|
|
Involucrin showed a similar evolution to
epithelial thickness in relation to culture time, but always with slight intensity as it
was observed as a weak lineal image.
An occasional finding in the first and second cultures (respectively 15 and 30 days old)
obtained from the same sample was the presence of a few keratohyaline granules in the
cells of the intermediate layer and in most superficial cells. This was not evident in the
other samples. A slight growth of non-epithelia] dermal connective tissue was observed in
one sample only (from a secondary culture). With respect to the control samples, foetus
and newbom skins presented the highest immunohistochernical expressivity (always more in
foetus skin) but not in the cell layers. The adult skin presents more cell layers
and less expressivity in basal and suprabasal layers but more superficial differentiation,
with high positivity to involucrin.
Discussion
A comparison of cell cultures with
control skin indicates that cultures a few days old are similar to foetus and newborn skin
with regard to immunohistochemical expressivity (but not as regards the number of cell
layers). Adult skin is more similar to secondary cultures in view of its superficial
differentiation, slight positivity to involucrin, and greater number of cell layers (the
cultures never in fact present as many cell layers as adult skin).
The number of cells derived from each primary tissue source that we obtained on day 8 was
approximately I x 101 cells/cml and after at least 30 days 12 x 101 celIS/CM2. These
values are in the range of cells obtained from each primary tissue source. When the
epidermal sheets were detached from the flasks by dispase, they presented an organization
and structure that were simpler and less differentiated than normal epidermis. The basal
layer was better preserved in secondary cultures, appearing as a single line, with cubic
or column-shaped cells.
The suprabasal cells were poorly organized and formed irregular layers varying in
thickness in the same cell and from sample to sample (2-7 cell layers). These cells were
prismatic, tending to flatten horizontally in the more superficial layers.
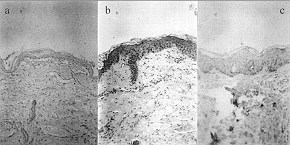 |
Fig. 2 - Histological sectoring:
a) foetus skin (29 weeks' gestation)
b) newborn skin
c) adult skin |
|
In the most external layer, the squamous
layer, the cultures showed one or two lines of flattened cells with nuclei and organelles
in variable states of degeneration.
| Case |
General
description |
AE1 |
AE3 |
5D3 |
Involuerin |
Foetal
skin |
Basal
layer+
2-3 intermediate
cell layers+
squamous layer |
+++ in
basallayer and
conjunctive glands |
+++ in
intermediate
zone and + in
squamous layer and conjunctive gland |
- |
Weak+ |
Newborn
skin |
Basal layer+
2-3 intermediate
cell layers+
squamous layer |
++ in
basallayer and
conjunctive glands |
++ in
intermediate
zone and + in
squamous layer and conjunctive gland |
- |
++ |
| Adult skin |
Basal layer+
2-3 intermediate
cell layers+
squamous layer |
Weak+ |
++ in
intermediate
zone and + in
squamous layer |
- |
+++ |
|
| Table III - Summary of the morphology and immunohistochemistry
of control skin samples |
|
In conclusion, cultured epithelial sheets
show a similar structure to that of the epidermis from which they are derived, even when
the stage of differentiation is not complete. The number of cell layers in the
intermediate layer is usually lower, the granulous layer is absent, and the superficial
cells show a lower level of keratinization. This partially differentiated sheet retains
its capacity of further differentiation when grafted on to the wound. 12-11 This is very
important for establishing a mechanical barrier and linking the grafted sheet to the
underlying connective tissue. When grafted, the resulting constituted epidermis forms a
barrier to bacterial penetration and dehydration.
RESUME. La couverture
cutanée des défauts gros et profonds de la peau dus aux brûlures ou à certaines
maladies présente un problème chir-urgique complexe. Les kératinocytes humains normaux
peuvent être cultivés in vitro en manière sérielle, et dans les conditions
appropriées ils produisent un épithélium pavimenteux stratifié. Bien que le
stratum corneuni ne se développe pas in vitro, ces cultures nous permettent
d'étudier les phases précoces du développement, de la prolifération, et de la
différentiation des kératinocytes humains. Pour mieux comprendre ces données, les
Auteurs ont étudié la viabilité et l'aspect histologique (analyse morphologique) et
immunohistochimique des lambeaux épidermiques, testés à divers moments de la culture,
en comparaison de la peau humaine normale obtenue de foetus, nouveauxnés, et adultes. Ils
ont observé que les cultures de quelques jours sont similaires à la peau des foetus et
des nouveaux-nés pour ce qui concerne l'expressivité immunohistochimique (mais non le
numéro des couches cellulaires). La peau des adultes est plus similaire aux cultures
secondaires pour ce qui concerne sa différemiation superficielle, sa légère positivité
à l'involucrine, et le numéro supérieur de couches cellulaires, même si les cultures
-ne présentent jamais un numéro tellement élevé de couches cellulaires comme la peau
des adultes.
BIBLIOGRAPHY
- Rheinwald J.G., Green H.: Serial cultivation of strain of
human epidermal keratinocytes: the formation of keratinizing colonies from single cells.
Cell, 6: 331-44, 1975.
- Green H., Kehinde 0., Thomas J.: Growth of cultured human
epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA,
76: 5665, 1979.
- Compton C.C., Gill J.M., Bradford D.A. et al.: Skin
regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days
to 5 years after grafting: a light, electron microscopic and inummohistochemical study.
Lab. Invest., 60: 600, 1989.
- De Luca M., Albanese E., Megna M. et al.: Human oral
epithelium reconstituted in vitro and transplanted onto patients presenting oral mucosa
defects retains properties of the original donor site. Transplantation, 50: 454, 1990,
- Franzi A.T., D'Anna F., Zicca A., Trabucchi E.:
Histological evaluation of human cultured epithelium before and after grafting. Burns, 18:
26-31, 1952.
- Daniels J.T., Kearney J.N., Ingham E.: Human keratinocyte
isolation and cell culture: a survey of current practices in the UK. Burns, 22: 35-9,
1996.
- Stairio-Coico L., Higgins P.J., Darynkiewicz Z. et al.:
Human keratinocyte culture. Identification and stating of epidermal cell subpopulations.
J. Clin. Invest., 77: 396-404, 1986.
- Galvin S., Loomis C., Manabe M., Dhouailly D., Sun T.T.:
The major pathways of keratinocyte differentiation as defined by keratin expression: an
overview. Adv. Dermatol., 4: 277-300, 1989.
- Fuchs E.: Epidermal differentiatiori: the bare essentials.
J. Cell Biol., 111: 2807-14, 1990.
- Bell E., Rosenberg M.: The commercial use of cultivated
human cells. Transplant. Proceed., 22: 971-4, 1990.
- Johnson E.W., Meunier S.F., Roy C.J., Parenteau N.L.:
Serial cultivation of normal human keratinocytes: a defined system for studying the
regulation of growth and differentiation. In Vitro Cell. Dev. Biol., 28A: 429-35, 1992.
- Green H.: The keratinocyte as differentiated cell type.
Harvey Lect., 74:101,1980.
- Andreassi L., Donali L., Malcovati M. et al.: In vitro
expanded human keratinocytes: clinical and biological advances. In: Galli C.L., Hensby
C.N., Marinovich M., "Skin Pharmacology and Toxicology. Recent Advances", Ser.
A., Life Science, Plenum 181: 257, 1989.
Hefton J.M., Amberson J.B., Biozes D.G.,
Weksler M.E.: Grafting of burn patients with allografts of cultured epidermal cells.
Lancet, 2: 428-30, 1983.
This paper was
received on 4 December 1996.
Address correspondence to: Dr Esther Garefa Fernandez
Dept. de Inrnunologfa, Hospital de Cruces, Plaza de Cruces s/n,
48903 Baracaldo, Vizcaya, Spain
Fax: +34.4.485 09 18
Acknowledgements. This study was supported by grants from the
Fondo de Investigaciones Sanitarias and the
Basque Health Service (93/0887 Ref). i |
|

