Annals of Burns and Fire
Disasters vol. XI n. 2 June 1998
VARIATIONS IN NUTRITIONAL
PARAMETERS AFTER THERMAL INJURY IN MAN
Bollero D., Giannotti L. Stella M.,Broglio F. Calcagni
M., Ghigo E. Magliacani G.
Department of Plastic Surgery, CTO
Burn Centre, Turin, Italy
Division of Endocrinology, Department of Internal Medicine, University of Turin
SUMMARY. In spite of optimized
artificial nutrition, the development of malnutrition is often rapid in critically ill
patients. This particularly applies to burn
patients, in whom dramatic metabolic and hormonal alterations occur. The aim of this study
was to define the variations of nutritional parameters, including IGF I levels, in burn
patients. To this goal, in 22 burn patients (mean age ± SEM, 46.5 ± 3.4 yr; BMI, 24.9 ±
0.9 M2; % burn surface area 26.0 ± 3.0%; ROI score, 0.22 ± 0. 1) we evaluated
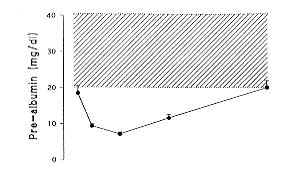
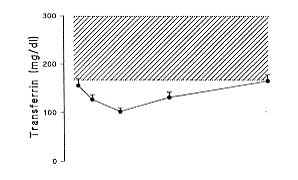
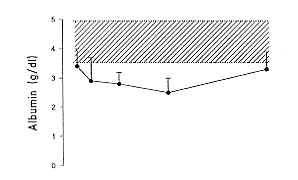
prealburnin (pre A), al burnin (A) and transferrin (TRA) as well as IGF I levels on days
1, 3, 7, 14 and 28. On day I post burn, pre A (18.5 ± 2.1 mg/dl), A (3.5 ± 0.2 g1l) and
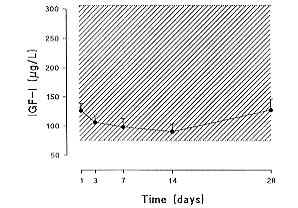
TRA (158.0 ± 13.0 mg/dl) were lower than the normal range, while IGF I levels were still
in the low normal range (120.2 ± 11.2 pg/1). Pre A, A and TRA underwent a further
decrease, with the lowest point on day 7, for pre A and TRA (7.1 ± 0.6 rng/dI and 103.0
± 6.5 mg/dI, respectively), and later, on day 14, for A (2.7 ± 0.2 g/dl). A rebound
increase was observed from day 7 for pre A and TRA until day 28 (20.3 ± 1.6 mg/dl and
165.5 ± 13.0 mg/dI, respectively), when these parameters were in the normal range. On day
28, A levels persisted lower than the normal range (3.0 ± 0.2 g/dl). IGF I levels showed
a progressive decrease until day 14 (86.9 ± 10.9 pg/1), when they were as low as in
hypopituitaric patients with severe growth hormone deficiency. IGF I levels then increased
and on day 28 (p < .05) they were again in the low normal range (127.4 ± 19.0 pg/1). A
levels, but not pre A and TRA levels, showed a negative correlation to burn extent and ROI
score (r = .07; p < .05) on days 3 and 7
only, while IGF I levels were not associated either with other nutritional parameters or
with burn extent and ROI score. These findings indicate that in burn patients pre A, A and
TRA levels, but not IGF I levels, are reduced within a few hours post burn. Pre A and TRA show a faster recovery and are
normal one month after thermal injury, when A levels still are reduced. IGF I levels show
a delayed decrease, which is as marked as in hypopituitaric patients with severe GH
deficiency, on day 14 post burn. One month after the burn lGF I levels are normalized.
These findings confirm the usefulness of IGF I as a marker of nutritional status, together
with al burnin, prealburnin and transferrin as a marker of nutritional status, although
only al burnin levels are associated with the severity of the injury and could have
prognostic value.
Introduction
Malnutrition rapidly occurs in
critically ill patients with sepsis and severe trauma and particularly following burns when dramatic metabolic and hormonal
alterations are present. In burn patients hypermetabolism associated with protein and fat
catabolism, negative nitrogen balance, hyperglycaemia and insulin resistance is a frequent
occurrence` and the response to nutritional support is only slight. The increase in
capillary permeability due to an extensive burn,
particularly when associated with sepsis, further impairs the degree of hypoproteinemia.
As a result, in burned patients, the progressive loss of body cell mass, particularly of
muscle mass, is a common development ......
The monitoring of nutritional status is thus of major importance in order to provide
adequate support. Preal burnin (pre A) is a sensitive marker of protein deficiency, as it
is a rapid turnover protein and its synthesis in the liver promptly reflects protein
deficiency and refeeding.
Serum al burnin (A) and transferrin (TRA) are other markers of nutritional status.
However, A is an insufficiently sensitive marker of rapid change in protein metabolism,
particularly when compared with pre A and TRA; in fact, both TRA and pre A reliably
reflect rapid changes in protein balance while A levels do not show significant
variations, even after prolonged protein restriction.
More recently, the evaluation of IG17 1 levels has received great attention as another
marker of nutritional status." IGF 1 is a hormone endowed with endocrine and
paracrine/autocrine action and its synthesis and release depend on growth hormone (GH)
secretion` and nutritional factors, mainly amino acids and glucose ...... Other hormones,
such as insulin, glucocorticoids, thyroid hormones and gonadal steroids, also influence
IGF 1.
Evidence that amino acids and glucose play a critical role in IG17 1 synthesis favoured
the hypothesis that this hormone could be a sensitive marker of nutritional status. 20
This hypothesis has been demonstrated` and IG17 1 is clearly reduced in malnourished,
diabetic and critically ill patients In cases of malnutrition, reduced IG17 1 synthesis
reflects peripheral resistance to the bioactivity of GH, the secretion of which is
frequently enhanced. 26 On the basis of these premises, this study defines variations in
nutritional parameters following thermal injury until day 28 post burn in patients
admitted to a burn unit receiving artificial nutrition in order to cover their caloric and
protein needs.
Patients and methods
The study was conducted in the
CTO Hospital Burn Centre in Turin on adult patients after thermal injury.
From July to December 1996, 22 subjects admitted to our burn centre following a major
thermal injury were considered for enrolment in the study. Patients considered eligible
for inclusion in the study were 20 60 yr of age and 80 110% of ideal weight, had no
history of hepatic, renal or cardiac failure or severe chronic disease, cancer disease,
diabetes mellitus or pre existing metabolic disorders, and were free of glucocorticoid
therapy and recent chemotherapy. The patients arrived at the burn unit within 24 h of
their burn injury. The ROI score 2 and burn surface area were determined on admission. The
initial treatment was intravenous rehydration therapy following the Parkland formula,
using lactated Ringers solution, after which artificial nutrition (entero parenteral)
commenced (40% carbohydrates, 35% fat and 10% protein: 8 10 g N). The patients received
insulin in order to maintain glucose levels lower than 180 mg/dl.
The total amount of calories was calculated following the Curreri formula (approximately
25 kcal/kg + 40 kcal/% burn).
Enteral nutrition with Osmolyte was initiated 6 8 h after admission to the burn unit.
After the second 24 h parenteral nutrition began via a central vein.
Blood and plasma products, al burnin, antibiotics and analgesics were administered when
needed. Of the 22 patients, 18 developed sepsis within the first 10 days postburn.
Clinical details of the patients are given in Table
I.
Patients were studied for 28 days after admission to the burn centre: observations were
recorded at five different times: within 24 h of admission (day 1) and on days 3, 7, 14
and 28 of burn unit stay.
On day 1 patients underwent the following:
- physical examination,
including determination of body mass index (BMI) and evaluation of protocol
inclusion/exclusion criteria
- calculation of ROI score
- laboratory tests, as reported
below
All laboratory tests were
repeated on days 3, 7, 14 and 28. Blood samples were collected through a central venous
access; in all patients the lines were prepared for clinical utilization, independently of
the study. The determinations included routine tests, such as complete blood count,
platelets and electrolytes, and nutritional parameters such as insulin like growth factor
1 (IGF 1), al burnin (A), preal burnin (pre A) and transferrin (TRA). The normal range
levels for pre A, TRA and A were 21 42 mg/dl, 168 302 mg/dl and 3.6 5.2 g/dl,
respectively, while the normal range levels for IGF 1, calculated in a population of 256
normal adults aged 20 60 yr, were 76320 pg/1. The values are expressed as mean ± SEM.
The statistical analysis of the data was carried out using the Kruskall Wallis ANOVA test
and the Mann Whitney U test where appropriate.
Results
On post burn day 1, pre A, A and TRA were lower than the
normal range (18.5 ± 2.1 mg/dI, 3.5 ± 0.2 g/l and 158.0 ± 13.0 mg/dl, respectively),
while IGF I levels were still in the low normal range (120.2 ± 11.2 pg/1).
Pre A, A and TRA underwent a further decrease, with a low point on day 7 for pre A and TRA
(7.1 ± 0.6 mg/dl and 103.0 ± 6.5 mg/dI, respectively) and on day 14 for A (2.7 ± 0.2
g/dl).
A rebound increase was observed from day 7 for preA and TRA until to day 28 (20.3 ± 1.6
mg/dI and 165.5 ± 13.0 mg/dI, respectively), when these parameters were in the normal
range. A levels remained lower than the normal range (3.0 0.2 mg/dl).
IGF I levels showed a progressive decrease up to day 14 (86.9 ± 10.9 pg/1), when they
were as low as in hypopituitaric patients with severe GH deficiency. IGF I levels then
increased and on day 28 (p < .05) they were again in the low normal range (127.4 ±
19.0 pg/1). The results are summarized in Fig. 1.
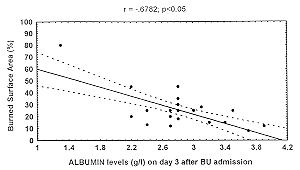
A levels (but not pre A and TRA levels), showed a negative correlation to burn extent and ROI score (r = .07; p < .05) on days 3 and 7 (Fig. 2) only, while IGF I levels were not
associated either with either other nutritional parameters or with burn extent and the ROI score.
Discussion
Our findings indicate that in the burn
patients we treated preal burnin, al burnin and transferrin levels (but not IGF 1 levels)
were reduced within a few hours of the trauma. Preal burnin and transferrin showed a more
rapid return to normal, within a month of the thermal injury, when al burnin levels were
still reduced. IGF 1 levels showed a delayed decrease which was as marked as that observed
in hypopituitaric patients with severe GH deficiency on day 14 post burn. IGF 1 levels
were normalized one month post burn. According to other studies, preal burnin, al burnin
and transferrin levels show a prompt reduction (within 24 h) after a thermal injury
and present a further reduction in the first week after admission to the burn unit.
Table 1 Clinical
details |
|
Sex |
Admission day |
Septic agents |
Age |
Weight |
H |
13M1 |
13urn(%) |
ROl score |
|
|
|
|
(yr) |
(k g) |
(m) |
(kg/m2) |
|
|
F. L. |
F |
03 10 1996 |
Staph. Aureus,
Sr. Epiderm. |
71 |
70 |
1,6 |
27,34 |
18 |
0,270 |
G.M.G. |
F |
22 10 1996 |
Str. Pneumoniae, Staphiloc. |
36 |
50 |
1,6 |
19,53 |
8 |
0,007 |
J.M. |
F |
31 10^1996 |
No sepsis |
22 |
56 |
1,68 |
19,84 |
20 |
0,007 |
L.C. |
F |
31 07 1996 |
Staph.Aureo,Pseudom. |
37 |
48 |
1,6 |
18,75 |
15 |
0,013 |
P.R. |
F |
14 09 1996 |
E.Coli,Enterococcus |
60 |
58 |
1,6 |
22,66 |
25 |
0,410 |
P. M. A. |
F |
18 08 1996 |
Staph. Aureus,
Pseudomon. |
65 |
75 |
1,55 |
31,22 |
25 |
0,320 |
R.G. |
F |
18 11 1996 |
Staph. Epid,
Xantomon. Malt |
64 |
83 |
|
|
25 |
|
R. 13. |
F |
15 10 1996 |
Staph.Aureus |
66 |
68 |
1,6 |
26,56 |
13 |
0,130 |
C.G.P. |
m |
31 08 1996 |
Staph. Aureus, Enteroc. Fec. |
42 |
85 |
1,8 |
26,23 |
25 |
0,070 |
F.G. |
m |
25 08 1996 |
No sepsis |
21 |
105 |
1,75 |
34,29 |
25 |
0,016 |
F.P. |
m |
19 11 1996 |
No sepsis |
67 |
75 |
1,7 |
25,95 |
80 |
0,99 |
G. M. |
m |
22 08 1996 |
Staph.Aureus |
46 |
82 |
1,74 |
27,08 |
35 |
0,320 |
G. A. |
m |
29 06 1996 |
Staph. Aureus |
23 |
80 |
1,73 |
26,73 |
28 |
0,021 |
G. L. M. |
m |
24 10 1996 |
Staph. Epidermis |
46 |
80 |
1,75 |
26,12 |
45 |
0,770 |
M.U. |
m |
04 07 1996 |
Staph.Aureus and
Epidermis |
28 |
60 |
1,8 |
18,52 |
17 |
0,007 |
M. A. |
m |
23 06 1996 |
Kebsiella |
61 |
78 |
1,78 |
24,62 |
25 |
0,330 |
N.C. |
m |
25 10 1996 |
Staph.Aureus |
47 |
94 |
1,75 |
30,69 |
18 |
0,033 |
P. M. |
m |
27 08 1996 |
No sepsis |
26 |
56 |
1,58 |
22,43 |
12 |
0,005 |
R. L. |
m |
30 07 1996 |
No sepsis |
54 |
70 |
1,75 |
22,86 |
30 |
0,210 |
R.C. |
m |
15 08 1996 |
Staph.Epid,Pseudomon. |
58 |
75 |
1,7 |
25,95 |
25 |
0,250 |
S.P. |
m |
12 07 1996 |
No sepsis |
30 |
68 |
1,75 |
22,20 |
20 |
0,093 |
T. D. |
m |
13 09 1996 |
Str. Pneum,
Sta ph. Aureus |
52 |
72 |
1,69 |
25,21 |
45 |
0,430 |
|
|
|
|
|
|
|
|
|
|
Mean |
|
|
|
46,45 |
72,18 |
1,69 |
4,99 |
26 |
0,22 |
SD |
|
|
|
16,12 |
13,66 |
0,08 |
4,02 |
is |
0,26 |
SEM |
|
|
|
3,44 |
2,91 |
0,02 |
0,88 |
3 |
0,06 |
|
|
This progressive reduction of
classical nutritional parameters is concomitant with the prompt development of the
hypercatabolism that characterizes the first week after thermal injury.2,4,2 As widely
demonstrated by previous studies, this first phase of the disease is characterized by a
marked impairment of nutrient uptake and utilization, leading to progressive loss of
muscle and 2 adipose mass. The loss of muscle mass occurs in spite of optimized and
enriched nutrition which normally prevent the loss of fat mass.
The monitoring of classical protein markers, such as al burnin and, in particular,
prealburnin and transferrin, is of major importance for the evaluation of nutritional
status. It also helps in the prediction of the outcome and in decisions regarding
therapeutical support.
Confirming evidence that metabolic status improves by the second week after burn unit admission, 2 prealburnin and transferrin
levels showed a progressive increase and reached normal levels on day 28. This means that
an optimized artificial nutrition had been provided, preventing prolonged protein
catabolism. In the present study, parameters of energy expenditure, such as indirect
calorimetry and the measurement of nitrogen balance, were not evaluated, and we were thus
unable to demonstrate a direct association between the progressive reduction of
hypermetabolism and protein marker improvement.
In agreement with evidence that al burnin is not a sensitive and prompt marker of changes
in protein metabolism, al burnin levels persisted below normal limits after 28 days and in
presence of normal preal burnin and transferrin levels. Al burnin levels are strongly
influenced by skin loss and infection and nearly all burn patients suffer sepsis.
The prognostic value of al burnin has been pointed out, mainly on the evidence that it
shows a close negative association with burn extent and the ROI score, at least in the
first week post burn. This evidence is clearly confirmed in the present study (Fig. 2), in
which no association was found with any other biochemical or hormonal parameter.
In the present study, it is interesting to note that a slight IGF 1 reduction was already
apparent on day 1, i.e. within 12 24 h of thermal injury, indicating IG17 1 as a sensitive
marker of rapid variation in nutritional balance, in agreement with other studies .4,12
IGF 1 progressively decreased until day 14, when the values were comparable with those of
hypopituitaric patients with severe GH deficiency, suggesting severe impairment of IGF 1
synthesis and release.
 |
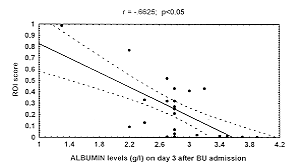 |
Fig. 2 Correlation al burnin/R01 score and al
burnin/ burned surface area on day 3. |
|
This finding implies that IGF
1 synthesis and release reflect an improvement in protein metabolism later than pre A and
TRA and that they depend on some other regulation mechanism.` This could be due to the
persistence of peripheral GH resistance" and/or to the marked impact of sepsis on IGF
1 synthesis In effect, some relationships between IGF 1 and cytokines such as interleukins
and TNF, which are strongly elevated during sepsis, have been reported ......IGF-I levels
progressively increased between days 14 and 28; IGF 1 levels on day 28 were in the low
normal range, at variance with A levels which were persistently reduced. This means that
adequate nutritional support had been provided and that GH secretion and sensitivity were
almost completely restored.
In conclusion, these findings confirm the usefulness of IGF 1, together with al burnin,
preal burnin and transferrin, as markers of nutritional status, although only A levels are
associated with the severity of the injury and could have prognostic value.
RESUME. La clinique des
Brûlures et de Chirurgie Plastique du Centre Hospitalier Universitaire de Tirana en
Albanie soccupe dun large éventail de maladies chirurgicales. Une grande partie de cette
activité concerne les séquelles des brûlures. Les Auteurs présentent une description
générale de cette activité pendant 1996, suivie par des observations sur le traitement
chirurgical des séquelles des brûlures. Après une comparaison entre les divers groups
des pathologies traitées avec des interventions chirurgicales, ils présentent limage
chirurgicale de leur clinique et discutent les conceptions médicales à la base dune
clinique de ce type. Les données statistiques indiquent clairement que la propagande
médicale, les recherches et le contrôle à long terme sont les moyens les plus efficaces
pour prévenir les brûlures et leur séquelles.
BIBLIOGRAPHY
- Meguin M.M., Brennan M.F., Aoki
T.T. et al.: Hormone substrate interrelationships following
trauma. Arch. Surg., 109: 776 83, 1974.
- Frayn K.N.: Hormonal control
of metabolism in trauma and sepsis. Clin. Endocrinol., 24: 577 99, 1986.
- Voerman H.J., Greneveld
A.B.J., de Boer H. et al.: Time course and variability of the endocrine and metabolic
response to severe sepsis. Surgery, 114: 951 9, 1993.
- Nygren
J., Sammann M., Malm M. et al.: Distributed anabolic hormonal patterns in burned patients:
the relation to glucagon. Clin. Endocr., 43: 49t 500, 1995.
- Nordestrom J., Carpentier
Y.A., Askanazi J. et al.: Free fatty acid mobilization and oxidation during total
parenteral nutrition in trauma and infection. Ann. Surg., 198: 725 35, 1983.
- Ming Yu Y., Young VR.,
Castillo L. et al.: Plasma arginine and leucine kinetics and urea production rates in burn patients. Metabolism, 44: 659 66, 1995.
- Nguyen T.T., Gilpin D.A.,
Meyer N., Hemdon D.N.: Current treatment
of severely burned patients. Ann. Surg., 223: 14 25, 1996.
- Pereira JL., Garrido M., Gomez
Cia T. et al.: Enteral nutrition in burn
patients. Nutr. Hosp., 7: 340 5, 1992.
- Golden
M.H.N.: Transport proteins as indices of protein status. Am. J. Clin. Nutf., 35: 1159 65, 1982.
- Wolfe R.R.: Nutrition and
metabolism in burns. Critical care, 7:
1923, 1986.
- Hemdon D.N., Curreri R.W.,
Abston S. et al.: Treatment of burns. Current problems in surgery, 2: 347 73, 1987.
- Abribat T., Brazeau P.,
Davignon I., Garret D.R.: Insulin like growth factor I blood levels in severely burned
patients: effects of time post injury, age of patient and severity of burn. Clin. Endocrinol., 39: 583 9, 1993.
- Jeejebhoy
K.N., Baker J.P., Wolman S.L. et al.: Critical evaluation of the role of clinical
assessment and body composition studies in patients with malnutrition and after total
parenteral nutrition. Am. J. Clin. Nutr.: 1117 27, May
1982.
- Ingenbleek Y., De Visscher M.,
De Nayer P.: Measurement of preal burnin as index of protein calorie malnutrition. Lancet,
106 9: July 15, 1972.
- Clemmons
D.R., Klibanski A., Underwood L.E. et al.: Reduction of plasma immunoreactive somatomedin
C during fasting in humans. J. Clin.
Endocrinol. Metab., 53: 1247 51, 1981.
- Isley W.L., Underwood L.E.,
Clemmons D.R.: Dietary components that regulate serum somatomedin C concentrations in
humans. J. Clin. Invest., 71: 175 82, 1983.
- Donahue
S.P., Phillips L.S.: Response of IGF I to nutritional support in malnourished hospital
patients: a possible indicator of short tenil changes in nutritional status. Am. J. Clin. Nutr., 52:
39 44, 1990.
- Jacob V., Le Carpentier J.E.,
Salzano S. et a].: IGF 1, a marker of undemutrition in haemodialysis patients. Am. J.
Clin. Nutr.,
52: 3944, 1990.
- Minuto
F., Barreca A.., Adami G.F. et al.: IGF I in human malnutrition: relationship with some
body composition and nutritional parameters. J. Parent. Ent. Nutr., 13: 392
6, 1989.
- Thissen J.P., Ketelstegers
J.M., Underwood L.F.: Nutritional regulation of insulin like growth factors. Endocr. Rev.,
15: 80 5, 1994.
- Furlanetto R.W.: Insulin like
growth factor measurements in the evaluation of growth hormone
secretion. Horm. Res., 33: 25 30, 1990.
- Clemmons D.R., Underwood L.E.:
Nutritional regulation of IGF I and IGF I binding protein. Ann. Rev. Nutr., 11: 393
412, 1991.
- Jones J.1., Clemmons D.R.:
Insulin like growth factors and their binding proteins: biological actions. Endocr. Rev.,
16: 3 34, 1995.
- Phillips
L.S., Unterman T.G.: Somatomedin activity in disorders of nutrition and metabolism. Clin. Endocrinol. Metab., 13: 145 80, 1995.
- Donaghy A., Ross R., Gimson A.
et al.: Growth hormone, insulinlike growth factor 1, and insulin like growth factor
binding proteinI e 3 in chronic liver disease. Hepatology, 21: 680 8, 1995.
- Jenkins R.C., Ross R.J.M.: The
endocrinology of the critically ill. Current opinion in endocrinology and diabetes, 3: 138
45, 1996.
- Ross
R.J.M.: Acquired growth hormone resistance. Eur.
J. Endocrinol., 132: 655 60, 1995.
- Roi L.D., Flora J.D., Davis
T.M. et al.: Two new burn severity indices.
J. Trauma, 23: 1023 30, 1983.
- Tredget E.E., Yu Y.M.: The
metabolic effects of thermal injury. World J. Surgery, 16: 68 79, 1992.
- Cammani F., Aimaretti G.L.,
Gianotti L. et al.: New approach to the diagnosis of GH deficiency in adults. Fur. J.
Endocrinology, 5: 122 34, 1996.
- Bentham J., Rodriguez Amao J.,
Ross R.J.M.: Acquired growth hormone resistance in patients with hypercatabolism. Horm.
Res., 40: 87 91, 1993.
- Dahn M.S., Lange M.P., Jacobs
L.A.: Insulin like growth factor I production is inhibited in human sepsis. 123: 1409 14,
1988.
- Peisen J.N., McDonnel K.J.,
Mulroney S.E., Lumpkin M.D.: Endotoxin induced suppression of the somatotropic axis is
mediated by interleukin I and corticotropin releasing factor in the juvenile rat.
Endocrinology, 136: 3378 90, 1995.
- Spath Schwalbe E.,
Schrezenmeier H., Bornstein S. et al.: Endocrine effects of recombinant interleukin 6 in
man. Neuroendocrinology, 63: 237 43, 1996.
| This paper was received on 30
March 1998. Address correspondence to: D.
Bollero, MD
Division of Plastic Surgery, burn Unit
CTO Hospital
Via Zuretti 29, 10126
Turin, Italy. |
|