| Annals
of Burns and Fire Disasters - vol. XI - n. 2 - June 1998
A RAT MODEL OF SMOKE INHALATION INJURY
Xie E.F., Yang Z.C., Wang D., Fu Q.F., Wei J.J.
Institute of Burn Research, Southwestern Hospital,
Third Military Medical University, Chongqing 400038, People's Republic of China
SUMMARY. Using a self-designed
apparatus consisting of a smoke generator and a traurnatogenic chamber, we successfully
established a rat model of smoke inhalation injury and observed smoke components and the
situation of animal pulmonary injuries. This model could be applied in experimental
research on the pathogenesis and treatment of inhalation injury.
Introduction
Although great advances in the prevention
and clinical management of burn shock and burn wound infection have enhanced the survival
of severely burned patients, the prognosis of large cutaneous burns complicated by
concurrent inhalation injury is still compromised. Smoke is among the most frequent
factors causing complicated inhalation injuries in clinical burned patients. As the
pathogenesis of smoke inhalation injury is not totally clear, and in view of the poor
specific methods available for effective treatment, inhalation injury remains a major
contributor to the morbidity and mortality of burn injuries and is badly in need of
further study.
Inhalation injuries are usually produced by gaseous and/or particulate products of
incomplete combustion. The generation of these toxic materials depends on conditions of
combustion such as oxygen supply, temperature, and heating rate in the fire.' In the light
of the specific condition that most inhalation injuries occur in a relatively closed
space, we designed and established a rat model of smoke inhalation injury for further
study of this injury on the basis of our original dog' and rabbit' models.
Patients and methods
- Model EU-2 electric respirator (made in Shanghai, China)
(Fig. 1). This connected the smoke generator (combustion chamber) with a rubber tube for
delivering smoke into the traumatogenic chamber.
- Auto-equilibrium recorder (Model SC-11, made in Sichuan,
China). This recorded the temperature in the smoke generator.
- Smoke generator. The combustion chamber was refitted from a
32 cm diameter portable pressure sterilizer. At a distance of 2 cm from the sterilizer
base along the wall, two 1.5 cm diameter holes were drilled, one for pulling through
electric lines, the other being a rubber tube I m in length and 1.5 cm internal diameter
connected to the respirator. A screw hole 1.5 cm diameter was bored on the sterilizer
cover to be fixed with a 10 em long iron pipe, which linked another rubber tube, 1.5 m
long and 1,5 cm internal diameter, to reach the traumatogenic chamber. At the base of the
generator, a 1200 W electric stove was installed, with an asbestos net over it.
- Traumatogenic chamber. The smoke chamber is a specially
made air-tight wooden rimless drawer with an inner area measuring 66 x 36 x 30 em. There
was a glass window 19 x 16 em set into the case ceiling to observe the rat in the
traurnatogenic chamber, as well as a thermometer and a sampling tube. Four 60 W
incandescent light bulbs were set up on the four corners of the ceiling in order to
maintain the air temperature and lighting in the traumatogenic chamber, which was
interconnected with the smoke generator by a rubber tube through a 1.5 cm diameter hole
drilled 2 em from the base of the case along the rear chamber wall.
- Smoking material. Dry pine sawdust (100 g, dried for 72 h
at 70 'C) was well mixed with 30 mI kerosene in a 24 em diameter stainless steel basin
before use.
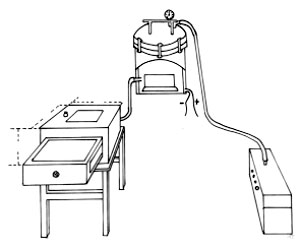 |
Fig.1 Sketch of induce smoke
inhalation injury. |
|
Forty adult healthy male Wistar rats
weighing 250-300g (supplied by the Experimental Animal Center of the Sichuan Institute of
Chinese Traditional Medicine) were divided into five groups. There were one control group
(n = 8) and four injured groups (n = 8 in each). The animals were anaesthetized with 2%
pentobarbital sodium (50 mg/kg) administered intraperitoneally, then settled in the dorsal
position. An intubation was placed in the right femoral artery, while another 2 mm
diameter silica gel tracheal intubation was inserted per os and well fixed
perorally. All the animals in the injured groups were injured with smoke inhalation 30 min
after operation, then observed and killed by bleeding respectively 2, 6, 12 and 24 h after
smoke inhalation injury. Control animals underwent similar procedures except that pure air
replaced smoke.
A Constantan thermistor-thennometer was used to record temperature. The electric stove in
the smoke generator was connected to the power at 220 V steady voltage, and preheated for
10-20 min with the cover. When the temperature at the point 1 cm below the sterilizer edge
registered 70 'C, the smoking material was placed into the combustion chamber and covered
tightly for 5 min before connecting the generator with the traumatogenic chamber. The
respirator was then switched on (600 ml/count and 24 counts/min) to drive air into the
smoke generator and at the same time to deliver smoke into the traumatogenic chamber,
where the air temperature was maintained at 38-40 'C. Five minutes later, when the chamber
was filled with smoke, the drawer of the chamber was opened swiftly, and each rat was
confined in the sealed traumatogenic chamber in a constant position, inhaling smoke for 2
min (reckoned by stopwatch). The rat was taken out of the chamber in order to breathe
fresh air for 5 min before being re-injured with smoke in the chamber for another 2 min.
The procedure was repeated three times, following the traumatogenic pattern 2-5-2-5-2
(min), ensuring that smoke inhalation lasted 6 min. During the whole procedure, the
electric stove in the smoke generator and respirator were at work continuously. The
arterial and tracheal incubations were removed 2 h after smoke exposure. On recovering
consciousness from anaesthesia, the animals were provided with food and water ad
libitum.
The smoke samples were collected from the traurnatogenic chamber 1 min before and I min
after each traumatization. The concentrations of oxygen and carbon monoxide in smoke
samples were measured using gas chromatography (Model SC-3, Sichuan, China) immediately
after collection.
The clinical signs of injured animals were observed. Arterial blood was collected from the
femoral artery respectively 5 min post-injury and prior to death. Arterial blood gases and
pH were determined by a Model AVL990 blood gas analyser made in Sweden. The anterior chest
wall was removed as soon as the animal was killed. In addition to observation of gross
pathological changes in the lung, samples taken from each low lobe of both lungs were
fixed in 10% neutral buffered fortnalin for 48 h and then embedded in paraffin, sectioned
at 6 [tm, stained with haematoxylin and eosin, and studied by light microscope for
micropathological changes. Total lung water JLW), extravascular lung water (EVLW) and
residual pulmonary blood water (RPBW) were measured according to the method of Noble et
al.
All the experimental results were presented as mean values ± standard errors. Data were
compared with normal controls by analysis of variance and t-test. Results were considered
significant if p < 0.05 (*) and highly significant if p < 0.01 (**).
Results
The oxygen and carbon monoxide
concentrations of smoke in the smoke chamber during the whole injury process appear in Table
1. The smoke chamber washermetically closed immediately each time after the animal was
put into it and/or taken out.
| |
Before injury |
During injury
for first time |
During injury
for second time |
During injury
for third time |
O2(%) |
17.58 ± 1.18 |
17.13 ± 1.39 |
16.89 ± 1.82 |
16.47 ± 1.38 |
CO (%) |
2.61 ± 0.29 |
2.52 ± 0.34 |
2.45 ± 0.40 |
2.36 ± 0.4 |
|
| Table 1 - Changes in
concentrations of oxygen and carbon monoxide in smoke chamber during whole traumatogenic
process (n = 20,,~ ± s) |
|
The clinical
manifestation in a few injured animals was apnoea during smoke inhalation. Most of the
injured animals showed an increased respiratory rate and hyperpnoea immediately after
injury. The respiratory rate then gradually decreased and dyspnoea occurred. In the late
stages, animals with smoke inhalation injury presented increased oronasal secreta, mouth
breathing, and wheezing. Table II presents the survival and death rate of animals
in each group.
| |
Control |
Hours
after smoke inhalation injury |
| |
- |
2 |
6 |
12 |
24 |
Total n° |
8 |
8 |
8 |
13 |
18 |
Survivors |
8 |
8 |
8 |
8 |
8 |
Deaths |
- |
- |
- |
5 |
10 |
|
| Table II - Survival and death rate in animals in
each group |
|
In addition to PaC02
slightly reducing due to hyperpnoea 5 min after injury, the main findings of arterial
blood gas analysis were progressively decreased pH, Pa02 and 02Cont and significantly
increased PaC02 and A-aD02. The metabolic acidosis was characterized by the results of
arterial blood gas analysis in the early stages after smoke inhalation injury, while
metabolic acidosis combined with respiratory acidosis was marked in the later stages (Table
III).
| |
Control |
Hours
after smoke inhalation injury |
| |
|
5/60 |
2 |
6 |
12 |
24 |
pH |
7.41 ± 0.04 |
7.11 ± 0.07** |
7.23 ± 0.18 |
7.29 ± 0.13** |
7.36 ± 0.12 |
7.27 ± 0.16* |
PaC02 (kPa) |
4.31 ± 0.49 |
3.16 ± 0.86 |
5.30 ± 1.95 |
6.32 ± 1.71** |
6.54 + 1.99** |
7.44 ± 1.23** |
1313 (mmol/L) |
44.63 ± 1.53 |
26.83 ± 2.58** |
36.24 ± 7.05* |
45.38 ± 2.89 |
46.50 ± 3.20 |
47.73 ± 5.80 |
HC03-(mmol/L) |
20.20 ± 1.78 |
7.52 ± 1.89** |
15.70 ± 4.66* |
25.13 ± 3.79** |
24.13 + 4.43 |
8.03 ± 3.99** |
StHC03- (MMOI/L) |
21.00 ± 1.05 |
9.26 ± 1.46** |
15.44 ± 4.63** |
21.53 ± 2.01 |
22.40 ± 2.59 |
23.70 ± 3.99 |
Pa02 (kPa) |
13.08 ± 0.79 |
7.82 ± 0.95** |
7.59 ± 0.87** |
7.55 ± 1.75** |
7.57 ± 2.18* |
6.09 ± 1.83** |
02Sat (%) |
97.40 + 0.67 |
76.31 ± 7.95** |
80.04 ± 14.84** |
82.79 + 14.44** |
86.42 ± 11.04** |
69.92 ± 0.55** |
02Cont (mmol/L) |
9.13 ± 0.08 |
7.14 ± 0.75** |
7.49 ± 1.40** |
7.73 ± 1.38** |
7.80 ± 1.67** |
6.52 ± 1.92** |
A-aD02 (kPa) |
1.04 ± 0.38 |
6.58 ± 1.77** |
5.14 ± 0.94** |
3.98 ± 1.14** |
3.35 + 1.22** |
3.00 ± 1.08** |
* p < 0.05,
** p < 0.01 |
|
| Table III - Results of arterial blood gas analysis (x ± s) |
|
Both TLW and EVLW
increased dramatically, peaking at 6 h and remaining high 24 h post-injury. No significant
change in RPBW was found in the five groups, indicating that RPBW did not influence the
changes of lung water content (Table IV).
| |
Control |
Hours after
srnoke inhalation injury |
| |
|
2 |
6 |
12 |
24 |
TLW (mi/g) |
2.08 ± 0.17 |
2.18 ± 0.21 |
3.14±0.54** |
2.67 ± 0.37 |
2.79 i 0.40** |
EVLW (rnllg) |
1.52 ± 0.07 |
1.59 ± 0.22 |
2.62 ± 0.53** |
2.14 ± 0.44* |
2.21 + 0.29** |
RM (mllg) |
0.49 ± 0.17 |
0.65 ± 0.22 |
0.52 ± 0.06 |
0.53 ± 0,16 |
0.58 ± 0.17 |
p
< 0.05, ** p < 0.01 |
|
| Table IV - Changes in lung water volume after injury x ± s) |
|
Gross
pathomorphological studies revealed congestion, oedema, necroses and exfoliation of the
tracheal mucosa, as well as congestion, swelling, and petechial and/or patchy haemorrhages
of lung tissues. Microscopically, haemorrhagic and oedematous cuffs around small blood
vessels and bronchioles with aggregation and infiltration of a large number of phagoeytes
were observed. The alveolar spaces were flooded with oedematous fluid and some red blood
cells (Figs. 2, 3).
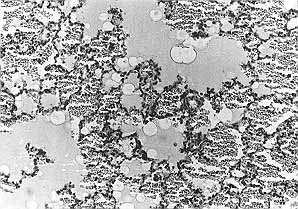 |
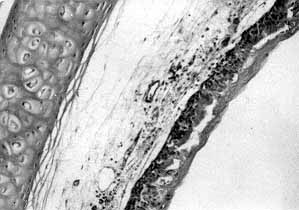 |
| Fig. 2 -
Six h post~injury, haemorrhage and swelling of lung tissues with aggregation and
infiltration of a large number of phagocytes were seen. The alveolar spaces were flooded
with oedematous fluid and red blood cells (HE x 400). |
Fig. 3 -
Twelve h post-injury, congestion, oedema, necroses and exfoliation of the tracheal mucosa
were observed (HE x 200). |
|
Discussion
The components of smoke are complex and
vary considerably, in relation to conditions of incomplete combustion, the combustion
materials, temperature, circumstances and timing. The inhalation of aldehydes, especially
acrolein - the major lesive substance contained in wood smoke and/or coated upon carbon
particles - may cause degeneration of airway mucosal proteins, loss of cilia and the
respiratory epithelium, and activation of alveolar macrophages, leading to inflammatory
reactions that result in damage to the alveolar capillary membrane, an increase in its
permeability, and pulmonary oedema.
We did not assay the contents of aldehydes in smoke but we determined the concentrations
of oxygen and carbon monoxide for the purpose of bringing the traurnatogenic conditions
and injury severity under control. It has been reported that carbon monoxide poisoning and
asphyxiation from hypoxia may be the main causes of fatalities in persons suffering from
smoke inhalation injury at the scene of an accident.' In the initial experiments when dry
pine sawdust only was used as combustion material, the concentration of oxygen detected in
smoke was only 15%, compared with that of carbon monoxide which was as high as 5%, a
figure directly associated with the high mortality of animals in the traumatogenic chamber
(30%) was a result of carbon monoxide poisoning and hypoxia. Kerosene, in contrast, is
considered to assist combustion and reduce the concentration of carbon monoxide and
aldehydes in smoke. The ratios of the concentrations of carbon monoxide, total aldehyde
and acrolein in kerosene smoke, in relation to those in wood smoke, are respectively
1:12.5, 1:20 and 1:50.' We conducted our experiment with a combination of wood sawdust and
kerosene as combustion material, which decreased mortality in the traumatogenic chamber
below 10%. The continuous measurement conditions showed well-controlled traumatogenic
conditions, as the concentrations of oxygen and carbon monoxide in smoke remained
consistent, and differences remained low throughout the traumatogenic procedure. Also, the
controlled temperature was maintained at 38-40 OC, preventing the thermal injury from
affecting the extent of the injury. If asphyxiation developed soon after injury, a 5 ml
syringe supported by artificial ventilation was helpful for resuscitation.
In the past, there have been two major two ways of establishing animal models of smoke
inhalation injury, i.e., perfusion by smoke via endotracheal intubation and smoking in a
traumatogenic chamber. The first is applicable in larger animals' and the second in
sirnaller ones.' In a preliminary test, we initially put the rats in a specially designed
cage. Conscious animals inhaled smoke freely in the traumatogenic chamber. Some animals
were found to struggle and present hyperpnoea, while others showed exhaustion and
breath-holding. These manifestations and others such as reflex closure of the glottis
because of smoke irritation may contribute to the varying amount of smoke inhaled, the
uncontrollable degree of injury, the remarkable variation in the extent of pulmonary
injury, and the high mortality (30%) in the traumatogenic chamber. We therefore proposed
an integration of two methods, in which endotracheal intubation was performed before the
effecting of smoke inhalation injury under narcosis in the traumatogenic chamber. This
integrated method kept the airways open, allowed relatively smooth respiration, and
diminished the factors affecting the amount of smoke inhaled, thus ensuring stability and
consistent injury.
The experimental results - clinical manifestations, arterial blood gas analysis,
determination of lung water content, and the pathological examination of lung tissues -
showed a number of changes in injured animals subjected to smoke inhalation according to
this model. The animals quickly developed acute pulmonary oedema and serious respiratory
failure; PaO- decreased progressively; PaC02 was clearly elevated; and lung water content
was markedly increased. Severe inhalation injury was also attested by pathological
examination. Pulmonary oedema peaked at 6 h and failed to return to control level 24 h
post-injury. These changes were consistent with the onset and development of severe smoke
inhalation lung injury and caused the predicted injuries. Of the 31 injured rats,
considered after 12 h and 24 h respectively, 16 animals survived and 15 died 12-24 h
post-injury, with a natural mortality of 48.4%, indicating that with this model mortality
at 24 h accounted for a near median lethal number (LD50). The model is therefore
applicable not only to the study of the pathogenesis of inhalation injury in the early
stages post-injury but also to research on early experimental treatment.
In conclusion, the rat model of smoke inhalation injury established in this study
satisfied three requirements for an ideal animal model, i.e., the acquisition of expected
injuries, stability and easy replication, and low mortality during traumatogenic
procedures. Owing to our limited facilities, we were unfortunately unable to determine and
control the content of acrolein, one of the main traumatogenic agents in wood smoke -
otherwise the model would be perfect.
RESUME. Les
Auteurs ont projeté un appareil composé d'un générateur de fumée et d'une chambre
traumatogénique pour créer un modèle murin de l'inhalation de fumée. Ils ont ainsi
observé les composantes de la fumée et la situation des lésions pulmonaires dans les
rats. Ce modèle pourrait être utilisé dans les recherches expérimentales sur la
pathogenèse et le traitement des lésions dues à l'inhalation.
BIBLIOGRAPHY
- Clark W.R.: Smoke inhalation: diagnosis and treatment.
World J. Surg., 16: 24-9, 1992.
- Sobel J.B., Goldfarb I.W., Slater H. et al.: Inhalation
injury: a decade without progress. J. Burn Care Rehabil., 13: 573-5, 1992.
- Terrill J.B., Montgomery R.R., Reinhardt C.F.: Toxic gases
from fires. Science, 200: 1343-7, 1978.
- Zhu P.F., Jiang K.Y., Chen F.M. et a].: Establishment of a
smoke inhalation model in dogs. J. Trauma (China), 3: 7-10, 1987.
- An J., Yang A.C., You Z.Y. et al.: A model for smoke
inhalation injury in rabbits. Acad. J. Third Military Med. Univ. PLA, 9: 71-3, 1987.
- Noble W.H., Obdrzalek J., Kay J.C. et al.: A new technique
for measuring pulmonary edema. J. Appl. Physiol., 34: 508-12, 1973.
- Prien T., Traber D.L.: Toxic smoke compounds and inhalation
injury: a review. Burns, 14: 451-60, 1988.
- Levine M.S., Radford E.P.: Fire victims: medical outcomes
and demographic characteristics. Am. J. Public Health, 67: 1077-80, 1977.
- Zirkia B.A., Ferrer J.M., Floch H.F.: The chemical factors
contributing to pulmonary damage in "smoke poisoning". Surgery, 71: 704-9,
1972.
Acknowledgements. The
authors would like to thank Ms Sumei Lin, Assistant of the Foreign Liaison Department,
MEBO International Group (USA) for participating in the preparation of the manuscript.
This study was supported by grants from the General Logistics Department of the Chinese
People's Liberation Army.
This paper was received on 28 December
1997.
Address correspondence to: Dr. Er-fan Xie
2069 Solaroso, Rowland Height, CA 91748, USA. |
G. WHITAKER
INTERNATIONAL BURNS PRIZE
PALERMO, ITALY
Under the patronage of the Authorities of
the Sicilian Region for 1999
By law n. 57 of June 14th 1983 the
Sicilian Regional Assembly authorized the President of the Region to grant the Giuseppe
Whitaker Foundation, a non-profit-making organization under the patronage of the Accademia
dei Lincei with seat in Palermo, an annual contribution for the establishment of the G.
Whitaker International Burns Prize aimed at recognizing the activity of the most qualified
experts from all countries in the field of burns pathology and treatment.
The amount of the prize is fixed at twenty million Italian lire. The prize will be awarded
every year by the month of June in Palermo at the seat of the G. Whitaker Foundation.
The Adjudicating Committee is composed of the President of the Foundation, the President
of the Sicilian Region, the Representative of the Accademia dei Lincei within the G.
Whitaker Foundation, the Dean of the Faculty of Medicine and Surgery of Palermo
University, the President of the Italian Society of Plastic Surgery, three experts in the
field of prevention, pathology, therapy and functional recovery of burns, the winner of
the prize awarded in the previous year, and a legal expert nominated in agreement with the
President of the Region as a guarantee of the respect for the scientific purpose which the
legislators intended to achieve when establishing the prize.
Anyone who considers himself/herself to be qualified to compete for the award may send by
January 3 1 st 1999 a detailed curriculum vitae to: Michele Masellis, M.D.,
Secretary-Member of the Scientific Committee G. Whitaker Foundation, Via Dante 167, 90141
Palermo, Italy. |
|


