Annals of
Burns and Fire Disasters - vol. XII - n° 4 - December 1999
SEVEN-YEAR EXPERIENCE IN THE
TREATMENT OF BURN PATIENTS WITH ALLOGENIC CULTURED KERATINOCYTES
Smirnov S.V.,¹
Vasiliev A.V.,² Paramonov B.A.,³ Loginov L.,¹ Kiseliov I.V.,² Danilova T.I.,²
Terskikh V.V.²
¹ Sklifosovsky
Research Institute of Emergency Care, Moscow, Russia
² Institute of Development Biology, Russian Academy of Sciences, Moscow
³ Military Medical Academy, St Petersburg
SUMMARY. This
study was conducted over a period of seven years (1992-98) in burn units at the
Sklifosofsky Research Institute of Emergency Care (Moscow) and the Military Medical
Academy (St Petersburg). Sixty transplants were performed in 48 patients with second- and
third-degree burns and donor sites. Allotransplantation was found to have a beneficial
effect on wound healing. We suggest that, after grafting, allogenic keratinocytes
activated by in vitro cultivation may produce a reparative environment necessary
for the proliferation and migration of recipient keratinocytes.
Introduction
Rapid closure of burn
wounds significantly improves the treatment of thermal injuries, but successful wound
coverage is often limited by a lack of suitable autografts.
Transplantation of cultured keratinocyte sheets is a promising approach in the restoration
of skin lesions after severe burns. Cultured keratinocyte allografts were first used as a
replacement of missing skin because it was thought that, in culture, keratinocytes did not
express HLADR. Later it was shown that cultured allografts are replaced by the recipient's
own cells. However, they offer certain advantages - immediate graft availability, an
almost unlimited supply, and bulk culture of keratinocytes. It is now evident that
allografts may function not only to replace skin but also to stimulate
re-epithelialization in the recipient's wound.
We first treated burns with cultured autologous grafts, after which we used cultured
allografts. We now report on our seven years of experience from 1992 to 1998 in allograft
treatment of burns.
Materials and methods
Wound preparation and
treatment
In serious burns necrectomy was performed in one or several steps. Wounds were treated
with antiseptics and water-soluble ointments. All patients were treated with antibiotics.
Chlorhexidine, hydrogen peroxide, and potassium permanganate were also used when
necessary. In the event of excessive granulation, hydrocortisone ointments were applied
(two cases). In two cases prednisolone was included in the treatment procedure in order to
suppress the immune reaction before and after grafting. Beginning 3-5 days before grafting
the wounds were bandaged daily.
Before transplantation the wounds were treated with gentamicin solution (0.16 g/1). In
three patients chlorhexidine solution was applied before gentarnicin treatment.
The wound bed was considered ready for grafting on the basis of the following criteria:
- presence of clean fine-granular reddish
granulation not elevated above the level of healthy tissues
- absence of Staphylococcus and Streptococcus
contaruination
- adhesiveness of wound bed
- marginal epithelialization
Keratinocyte culture
Allogenic keratinocytes were isolated from skin fragments taken from healthy patients
undergoing plastic surgery and cultured according to the method described by Rheinwald and
Green (with some modifications). In brief, epidermal keratinocytes were isolated by
overnight trypsinization at 4° C followed by additional treatment with diluted trypsin.
The complete medium consisted of DMEM:F12 (1:1) mixture supplemented with 10% foetal calf
serum (FCS), 10 ng/rnI epidennal growth factor (EGF) (Sigma), 5 ng/ml insulin (Sigma), 106
M isoproterenol (Sigma), and 5 ng/ml sodium selenite (Sigma). Keratinocytes were
cultivated without feeder layer in 75 cm² flasks (Costar) coated with collagen. The
medium was changed every other day and cultures were kept at 37° C in a humid atmosphere
incubator containing 3% CO,. The cultures became confluent within 3 to 4 weeks.
Transportation of
cultured sheets to hospital
Confluent epithelial sheets were either used instantly or stored at 26 'C until use.
The day before transplantation the culture medium was changed for the complete medium
without FCS. The culture flasks filled with Eagle MEM were delivered to the hospitals in
thermostatic containers.
Keratinocyte grafting
Keratinocyte cultures suitable for grafting were treated with 1.2 U/ml Dispase 11
(Sigma) in serum-free medium. Dislodged sheets, fixed on Paranett or Jelonet dressing
(Smith and Nephew), were grafted on prepared wounds pre-treated with gentarnicin in normal
saline solution (0. 16 gJ). The dressing was covered over with gauze moistened with the
same solution.
The first change of dressing depended on wound conditions. Usually it was performed on day
3-5 postgraft. Further dressings were done every second or third day. In cases where the
grafted epithelial sheet was visible on the wound surface, the wound was dressed with
Paranett or Jelonet and the gauze was moistened with gentamicin solution. When the
epidermal sheet was not seen on the wound surface but pronounced marginal
epithelialization was observed, dressings with antiseptics and water-soluble ointments
were applied to prevent bacterial contamination. In cases where visual inspection did not
reveal either the grafted epithelial sheet or marginal epithelialization we repeated
grafting with allogenic cultured sheets or with split-thickness transplants.
Histological analysis
Small biopsies obtained from grafted sites were fixed in alcohol-acetic acid and
embedded in paraffin, after which 5 [tm-thick sections were stained with haematoxylin and
eosin.
Results
Sixty transplantations
were performed in 48 patients at the Sklifosovsky Research Institute of Emergency Care and
the Military Medical Academy. The transplantations were carried out two weeks to three
months after injury in second- and third-degree burns and in donor sites.
Thirty allogenic grafts were performed in 25 patients at the Sklifosovsky Research
Institute of Emergency Care (SRIEC). In one patient the same wound was grafted twice. In
four patients the transplantations were performed in two successive stages. Twenty-three
patients were grafted with allogenic cultured epithelial sheets at the Military Medical
Academy (MMA). In two patients grafting was performed in three stages, and in three
patients in two stages.
The presence of the epidermal sheet on the wound bed during the first dressing change
provided considerable support for the success of the grafting. In practice, epidermal
sheet lysis was usually observed during the first rebandaging. We rarely observed total
rejection of the sheet after temporary take. Fig. I shows the distribution of results
according to these criteria. At SRIEC sheets were visible on the wound in 15 cases (50% )
during the first rebandaging.
Lysis occurred later in three cases. Partial lysis of the sheets was observed in six cases
(20%) and total lysis in nine cases (30%). At MMA these parameters corresponded
respectively to 13 cases (43%), 14 cases (47%), and 3 cases (10%).
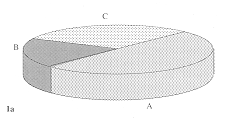
la - Sklifosofsky Research Institute
of
Emergency Care
|
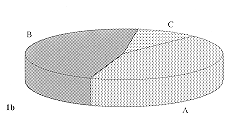
1b - Military Medical Academy.
|
Fig.
1 - Presence of transplanted epidermal sheets during first rebandaging.
(A - No lysis. B - Partial lysis. C - Total lysis) |
|
Lysis of
grafted epithelial sheets after the first dressing was observed more frequently in
third-degree than in second-degree burns or donor sites (Table 1).
Presence
of epidermal
sheets during
first rebandaging |
Donor
sites |
II-degree
burns |
III-degree
burns |
| |
SRI* |
MMA* |
SRI |
MMA |
SRI |
MMA |
No lysis |
2
(100%) |
3
(37.5%) |
6
(100%) |
8
(50%) |
7
(32%) |
2
(33.3%) |
Partial
lysis |
- |
5
(62.5%) |
- |
7
(44%) |
6
(27%) |
2
(33.3%) |
Total
lysis |
- |
- |
- |
1
(16%) |
9
(41%) |
2
(33.3%) |
Total |
2 |
8 |
6 |
16 |
22 |
6 |
* SRI
= Sklifosofsky Research Institute of Emergency Care
** MMA = Military Medical Academy |
|
Table
I - Presence of grafted epidermal sheets during the first rebandaging
(depending on burn thickness) |
|
At SRIEC
two allogenic transplantations were performed on donor sites and six on partial-thickness
burns. Epithelial sheets were visible during the first dressing change in all cases. Out
of 22 allogenic sheets grafted onto full-thickness burns the transplants were visible
during the first change of dressing in only seven cases (32%).
We found that the main causes of grafting failures were manipulation errors during the
procedure of grafting and further treatment of patients, the unsatisfactory condition of
granulation tissues, and the development of wound contamination.
The application of allogenic sheets in patients with second-degree burns was intended to
decrease the duration of treatment. When correct grafting was performed soon after burn
trauma on prepared wounds, restoration of the epidermis occurred 3-12 days earlier than
with routine treatment.
The maximum area of restored epidermis using allogenic sheets was 450 cm² at SRIEC and
450 cm² at MMA.
In patients with full-thickness burns, grafting of allogenic keratinocyte sheets can take
the place of routine split~thickness skin autografting in some cases. Table II summarizes
our data when full or partial skin restoration after allogenic keratinocyte grafting onto
limited areas of full-thickness burns was observed.
Skin
restoration |
Sklifosovsky
Military
Research Institute |
Military
Medical Academy |
Cornplete
skin restoration |
7 (32%) |
2 (333%) |
Partial skin
restoration |
7 (32%) |
2 (33.3%) |
Skin not
restored |
8 (36%) |
2 (33.3%) |
Total |
22 |
6 |
|
Table II
- Skin restoration after grafting of allogenic keratinocyte sheets in
patients with full-thickness burns |
|
In patients
with third-degree burns 22 transplantations were performed at SRIEC. Complete skin
restoration without split-thickness skin autografting was achieved in seven cases (32%).
It is interesting to note that in two patients the epidermal sheet was invisible during
the first rebandaging and that take of the graft was temporary in one patient. Restoration
of skin in these three patients was complete due to strongly induced marginal
epithelialization. The maximum area of restored skin was 300 cm². Partial skin
restoration was observed in six cases, while in one case the transplant sheet could not be
observed during the first rebandaging. In eight cases (36%) skin was not restored. At MMA
complete skin restoration in patients with full-thickness burn was achieved in two cases
(33.3%) without split-thickness autografting and partial restoration in two cases, with
failure in two cases. The maximum area of restored skin was 70 cm².
Case reports
Case 1. A
69-yr-old female arrived at SRIEC with flame burns in the back, the right buttock and part
of the hip, and the right lateral part of the body. She had sustained 20% TBSA burns (9%
full-thickness). She was considered to be in a bad condition because of her age, burn
area, and associated diseases (mitral insufficiency, cholelithiasis, chronic pancreatitis,
psoriasis, frequent pneumonia). She was given appropriate medical therapy. By day 33 of
treatment the area of granulation tissue was about 4% TBSA. Successive necrectomies were
performed. The burn wounds were treated conservatively because the patient refused
surgery. On day 50 post-trauma granulations remained in 1.5% TBSA. The granulation tissue
was fine granular, pale pink, and with scant wound fluid. By day 51 1.5% TBSA was covered
with allogenic cultured sheets. The first wound inspection was performed three days
post-graft. Pronounced marginal epithelialization was recorded. The wound area decreased
by one-third, but grafted sheets could not be seen. By day 6 post-graft we observed
progressive wound decrease owing to marginal epithelialization. By day 10 post-graft the
wound area amounted to 3 cm². By day 15 post-graft (day 66 post-burn) the skin was
completely restored.
Case 2. A 38-yr-old
male was hospitalized in the MMA Burn Unit with burns in the head, neck, trunk, and limbs.
The patient had sustained 50% TBSA flame burns (20% full-thickness skin loss). The patient
was given appropriate proper fluid and antibiotic therapy. The wounds were prepared for
autograffing intensively by necrolytic drugs. By day 16 post-injury mesh split-thickness
autografting onto full-thickness burns was performed. Partial-thickness wounds of the
trunk (300 cm²) and arms (150 cm²) were covered with allogenic cultured keratinocyte
sheets. Restored skin was observed on day 6 post-graft on the Dearly complete graft area
during the first dressing change. The grafted epithelium looked like a dim film. Biopsy
samples of reconstructed skin were taken on day 7 post-graft. The epidermis contained
10-13 cell layers and was loosely connected with underlying granulation tissue. The basal
cells exhibited destructive alterations, while the prickly cells appeared normal. The
partial-thickness wounds not treated with keratinocytes healed 7-12 days later. ne patient
was discharged from hospital on day 54 post-lesion. Skin biopsies were made in the
allograft area two years after treatment. The histological examination revealed a
well-developed epidermis with an almost straight border between dermis and epidermis. The
deep skin appendages were preserved.
Discussion
The use of cultured
allografts has significantly extended current knowledge of the regenerative biology of
skin grafts. Allogenic keratinocyte sheets have been shown to promote healing in a wide
variety of clinical instances, such as burns, leg ulcers, Lyell's syndrome, recessive
dystrophic epidermolysis bullosa, chronic otorrhoea, and donor sites.
Originally it was thought that cultured allografts acted as a "biological"
dressing and skin replacement. Today it is known that cultured grafts can also work as a
pharmacological agent.
The grafting of allogenic keratinocyte sheets leads not to permanent but to slightly
prolonged graft survival.. The use of specific probe for Y chromosome and DNA
fingerprinting of the wound covering epidermal cells taken after successful allografting
showed that keratinocytes originated from the recipient and not the donor cells. Zhao et
al. employed two methods to identify the presence of allografted keratinocytes on the
wounds: an indirect enzyme-conjugated Staphylococcus protein A to detect A or B blood
groups and a polymerase chain reaction to amplify the Y chromosome-specific DNA sequence.
It was concluded that the survival time of cultured allografts was prolonged up to 35 days
and that recipient tissue and graft tissue combined to provide coverage of mixed host and
donor cells. Moll et al. used a skin organ culture model for epidermal healing to
investigate the fate of transplanted keratinocytes. It was shown that autologous as well
as allogenic transplanted keratinocytes were integrated into the regenerating epidermis.
Relatively late rejection of allogenic keratinocyte grafts with no clinically visible
inflammation reaction suggests a temporary take of allogenic keratinocytes. There are data
suggesting that the effects of cultured allografts on wound healing may be
growth-factor-mediated as keratinocytes produce TGF-a and TGF-b. Cultured keratinocytes synthesize fibronectin and
collagen IV which stimulate the spreading and proliferation of keratinocytes. Allogenic
keratinocytes also produce interleukins.
It has been shown that allogenic grafts produce healthylooking granulation tissue, induce
the edge effect, and accelerate the shift of the epidermis from an inflammatory to a
regenerative state. Cultured keratinocytes also have antibacterial activity. It is
therefore evident that allografts exert different influences on the process of wound
healing that induce the proliferation and migration of recipient keratinocytes and
stimulate assembling of the basal membrane before they are rejected. Compton et al.
studied the reconstruction of skin in patients treated with cultured autologous
keratinocytes. Six days after grafting the process of de novo fon-nation of the
dermal-epidermal junction had begun and within 3-4 weeks the formation of mature
hemidesmosomes and the dermal-epidermal junction was complete. Cultured keratinocytes
undergo "activation", which includes increased cell attachment, spreading, and
migration as well as metabolic changes. We therefore suggest that culture-activated
allogenic keratinocytes stimulate the restoration of skin after transplantation onto the
wound bed.
Besides immediate graft availability and unlimited supply, allogenic keratinocytes
accelerate wound healing and produce excellent cosmetic results. The great advantage of
cultured allografts is the possibility of storing them for long periods of time in skin
banks.
Since the classical method of keratinocyte cultivation was described by Rheinwald and
Green, various other methods have been proposed. To facilitate grafting, keratinocytes
were cultivated on a polymer film. The growing of cells on micro-carriers is the most
promising method of cultivation that is suitable for the storage of cultured keratinocytes
in skin banks.
Conclusion
Although the role of
keratinocyte allografts in the acceleration of wound healing is not completely understood,
the use of allografts has led to a fascinating variety of medical applications. Allografts
are replaced by the recipient's keratinocytes but they stimulate wound healing. We suggest
that, after grafting, the allogenic keratinocytes activated by in vitro cultivation
produce the reparative environment necessary for the proliferation and migration of
recipient keratinocytes. The most significant advantage of using allografts is their ready
availability and the possibility of cryopreservation in skin banks.
RESUME. Cette
étude a été effectuée pendant une période de sept années (1992-1998) dans les
Unités de Brûlures à l'Institut de Recherches de la Thérapie d'Urgence Sklifosovsky
(Moscou) et à l'Académie Médicale Militaire (Saint-Pétersbourg). Soixante
transplantations ont été effectuées dans 48 patients atteints de brûlures de deuxième
degré et dans les sites donneurs. L'effet positif de l'allotransplantation sur la
guérison des lésions a été observé. Selon les Auteurs, après la greffe, les
kératinocytes activés par la culture in vitro créent l'environnement réparateur
nécessaire pour la prolifération et la migration des kératinocytes receveurs.
BIBLIOGRAPHY
- Terskikh V.V., Vasiliev A.V.: Cultivation
and transplantation of epidermal keratinocytes. Intern. Rev. Cytol., 188: 41-72, 1999.
- Morherm V.13., Benike C., Cox AJ. et al.:
Cultured human epidermal cells do not synthesize HLA-DR. J. Invest. Dermatol., 78: 32-7,
1982.
- Hefton LM., Amberson LB., Biozes D.G., Weksler M.E.: Loss of HLA-DR expression of human
epidermal cells after growth in culture. J.
Invest. Dermatol., 83: 48-50, 1984.
- Aubbck J., Irschick E., Romani N. et al.: Rejection, after a slightly prolonged survival time, of Langerhans cell-free
allogenic cultured epidermis used for wound coverage in humans. Transplantation, 45:730-7,
1988.
- Oliver A.M., Kaawach W., Mithoff E.M. et al.: The differentiation and proliferation of
newly formed epidermis on wounds treated with cultured
epithelial allografts. Br. J. Dermatol., 25: 147-54, 1991.
- Malakhov S.V., Vasiliev A.V., Paramonov B.A., Bautin E.A., Terskikh V.V.:
Autotransplantation of keratinocytes grown in vitro for treatment of extensive
burns. Vestnik Khirurgii im. I.I. Grekova, 4:
59-62, 1993 (Russian).
- Malakhov S.V., Paramonov B.A., Vasiliev A.V., Terskikh V.V.: Preliminary report of
clinical use of cultured allogenic keratinocytes.
Burns, 20: 463-6, 1994.
- Rheinwald J.G., Green H.: Serial cultivation of strains of human epidermal
keratinocytes: The formation of keratinizing colonies from single cells. Cell, 6: 331-44, 1975.
- De Luca M., Albanese E., Bondanza S., Megna M., Ugozzoli L., Molina F. et al.:
Multicentre experience in the treatment of burns with autologous and allogerric cultured
epithelium fresh or preserved in a frozen state. Burns, 15: 303-10. 1989.
- Madden MR., Finkelstein J.L., Staiano-Coico L. et al.: Grafting of cultured allogenic
epidermis in second- and third-degree burn wounds
on 26 patients. J. Trauma, 26: 955-62, 1986.
- Phillips T.J., Gilchrest B.A.: Clinical application of cultured epithelium. Epith. Cell. Biol., 1: 39-46, 1992.
- De Luca M., Albanese E., Cancedda R., Viacava A., Faggioni A., Zan Bruno G. et al.:
Treatment of leg ulcers with cryopreserved allogenic cultured epithelium: A multicentre
study. Arch. Dermatol.: 128: 633-8, 1992.
- Teepe R.G.C., Roseeuw D.I., Hermans J., Koebrugge E.J., Altena T., De Coninck A. et al.:
Randomized trial comparing cryopreserved cultured epidermal allografts with hydrocolloid
dressing in healing chronic venous ulcers. J.
Am. Acad. Dermatol., 29: 982-9, 1993.
- Napoli B., D'Arpa N., Masellis M. et al.: Use of cultured homologous keratinocytes in
the local treatment of Lyell's syndrome. Ann.
Burns and Fire Disasters, 9: 163-7, 1996.
- McGuire J., Birchall N., Cuono C., Moellman G., Kuklinska E., Langdon R.: Successful
engraftment of allogenic keratinocyte cultures in recessive dystrophic epidermolysis
bullosa. J. Invest. Dermatol., 88: 506A,
1987.
- Somers T., Verbeken G., Vanhalle S. et a].: Treatment of chronic post-operative
otorrhoea with cultured keratirocyte sheets. Ann. Orel. Rhinol. Laryngol., 106: 15-21, 1997.
- Teepe R.G.C., Koch R., Haeseker B.: Randomized trial comparing cryopreserved cultured
epidermal allografts with tulle gras in the treatment of split-thickriess skin graft donor
sites. J. Trauma, 35: 850-4, 1993.
- Duinslaeger L.A.Y., Verbeken G., Vanhalle S., Vanderkelen A.: Cultured allogenic
keratinocyte sheets accelerate healing compared to Op-Site treatment of donor sites in
burns. J. Burns Care Rehabil., 18: 545-51,
1997.
- Kirsner R.S., Falanga V., Kerdel F.A., Katz M.H., Eaglstein W.H.: Skin grafts as
pharmacological agents: Pre-wounding of the donor site. Br. J. Dermatol., 135: 292-6, 1996.
- Rouabhia M.: Skin regeneration after heterologous epidermal substitute grafting. Ann.
Burns and Fire Disasters, 10: 98-104, 1997.
- Brain A., Purkis P., Coates P. et al..:
Survival of cultured allogenic keiatinocytes transplanted to deep dermal bed assessed with
probe specific for Y chromosome. Br. Med. J., 298: 917-9, 1989.
- Van der Merve A.E., Mattheyse F.J., Bedford M. et al.: Allografted keratinocytes used to
accelerate the treatment of burn wounds are replaced by recipient cells. Burns, 16: 193-7,
1990.
- Zhao Y.B., Zhao X.-F., Li A. (Ngao) et al.:
Clinical observations and methods for identifying the existence of cultured epidermal
allografts. Burns, 18: 4-8, 1992.
- Moll L, Houdek P., Schmidt H., Moll R.:
Characterization of epidermal wound healing in a human skin organ culture model:
Acceleration by transplanted keratinocytes. J. Invest. Dermatol., 111: 251-8, 1998.
- Coffey R.J., Derynck R., Wilcox J.N. et
a].: Produduction and autoinduction of transforming growth factor a in human
keratinocytes. Nature (Lond.), 328: 817-20, 1987.
- Partridge M., Green M.R., Langdon I.D.,
Feldman M.: Production of TGF-a and TGF-P by cultured keratinocytes, skin, and oral
squamous cell carcinomas - potential autocrine regulation of normal and epithelial cell
proliferation. Brit. J. Cancer, 60: 542-8, 1989.
- O' Keefe E.J., Woodley D.T., Castello G. et al.: Production of soluble and
cell-associated fibronectin by cultured keratinocytes. J. Invest. Dermatol., 82: 150-5,
1984.
- Ohkura K., Fujii T., Konishi R., Terada H.:
Increased attachment and confluence of skin epidennal cells in culture induced by ascorbic
acid: Detection by permeation of trypan blue across cultured cell layers. Cell Struct. and
Funct., 15: 142-50, 1990.
- Woodley D.T., Wynn K.C., O'Keefe E.J.: Type
IV collagen and fibronectin enhance human keratinocyte thymidine incorporation and
spreading in the absence of soluble growth factors. J. Invest. Dermatol., 94: 139-43,
1990.
- Partridge M., Chantry D., Turner M. et al.:
Production of interleukin- I and interleukin-6 by human keratinocytes and squamous cell
carcinoma cell line. J. Invest. Dermatol., 96: 771-6, 1991.
- Moll L, Schonfeld M., Jung E.C.: Applikation von Kerafinozyten in der Therapic von Ulcera crumm. Hautarzt, 105:
548-52, 1995.
- Lang E., Schaefer E.M., Eickhoff U., Hohl
H.-P., Kramer M.D.: Rapid normalization of epidermal integrin expression after
allografting of human keratinocytes. J. Invest., Dermatol. 107: 422-7, 1996.
- Maier K., Ehrhard G., Frevert J.:
Antibacterial activity of cultured human keratinocytes. Arch. Dermatol. Res., 284: 119-21,
1992.
- Grinnell F.: The activated keratinocyte.
Upregulation of cell adhesion and migration during wound healing. J. Trauma, 30: 144-9,
1990.
- Burt A.M., Clarke J.A.: Transfer of autologous keratinocytes grown on a polymer reduces
time taken from biopsy to graft. Ann. Burns and Fire Disasters, 10: 215-8, 1997.
Vasiliev A.V., Smimov
S.V., Ermolinsky I.I., Zaikonnikova A.P., Samarova A.V., Kiseliov LV., Terskikh V.V.:
Restoration of epithelia] lesions by transplantation of human allogenic keratinocytes.
Intern. European A.I.R.R. Conference, Cologne, Germany, 44, 1997.
| This paper was received on 13 July
1999. Address correspondence to:
Dr. S.V. Smimov
Sklifosovksy Research Institute of Emergency Care
Moscow, Russia. |
|

