Annals of
Burns and Fire Disasters - vol. XIII - n. 2 - June 2000
THE EFFECT OF THE DELAY PHENOMENON ON THE ZONE OF STASIS IN BURNS: AN
EXPERIMENTAL STUDY IN RABBITS
lsik S, Kopal C., Karacalioglu
O., Selmanpakoglu N.
Department of Plastic and Reconstructive Surgery, Gu1hane Military Medical Academy,
Ankara, Turkey Department of Nuclear Medicine, G01hane Military Medical Academy
SUMMARY. Twenty-five New Zealand white rabbits (weight
3.0 ± 0.5 kg) were used to study the destiny of skin responding to ischaernia in
experimental burns. In the first group, dorsal delay flaps (4 x 10 cm) with the caudal
pedicle (N°5) were elevated in two stages and sutured to their donor sites. On day 7 of
elevation, dorsal acute flaps the same size were elevated counterlaterally to the delay
flaps. The extent of the distal necrotic areas was calculated one week later. In the
second group, delay flaps were elevated and rows of burns were created in the distal half
of the flap (N°10). A probe (0.5 x 1 cm of each) was heated in boiling water and applied
four times on the skin for 20 sec in such a fashion that a cross-shaped interspace area
(0.5 cm, in width) was achieved. The same pattern of burn was created on the normal skin
next to the flap. In the third group, delay flaps and acute flaps were elevated in the
abovementioned fashion and the same pattern of burn was created in the distal halves of
the flaps. Tests, including laser Doppler flowmetry, nuclear irnaging, and
autoradiography, were performed on days 1 and 7 post-burn. In the first group, the average
necrotic area in the acute flaps was 310.4 mm2, and all the delayed flaps
survived. Burn rows were then created 2 cm proximally to the distal edges of the flaps in
groups 2 and 3. In group 3, the distal necrosis of the acute flaps was significantly
increased to 774 ± 138 mm2 on day 7. The laser Doppler flowmetry measurements
revealed decreased distal circulation in the acute flaps, and 4.5 ± 2.2 perfusion units
in the delay flaps on day 7 post-burn. The amount of necrotic change in burned areas in
group 2, as calculated from autoradiographies, was found to be 316 ± 62 mm2 in
normal skin and 274 ± 45 mm2 in delay flaps on day 7. In group 3 the amount
was 288 ± 54 mm2 in delay flaps and 680 ± 41 mm2 in acute flaps.
To conclude, it seems that the mechanism involved in the saving of ischaemic tissues does
not significantly save the zone of stasis in burns.
Introduction
The zone of stasis is
the part of the burn wound that includes not only the central area of the burn wound (when
present) but also the most peripheral hyperaemic reaction in the surrounding tissues. The
metabolic and infectious reactions occurring in this area usually lead to further
necrosis, which results in increased burn width and depth. The saving of the zone of
stasis and the understanding of the pathophysiological events in this region have been the
recent goal of burn research. Many researchers have reported that this zone can be
saved.The events occurring in this zone are defined by some authors as "progressive
burn ischaemia".
Numerous reports have focused on ways of increasing the tissues' tolerance of ischaemia.'
As the understanding of the physiology of tissue ischaemia has progressed, some reports on
the use of newly discovered chemical mediators have appeared. However, as these chemical
materials have been invented, there have also been reports in burns literature regarding
the same chemicals used to save the zone of stasis. The delay phenomenon is the only
method that is generally accepted in clinical practice to increase the ischaernic
tolerance of the flap tissue. The mechanism most often suggested for the delay phenomenon
is the opening and patency of choked vessels which have been demonstrated histologically.
In a recent study, we demonstrated experimentally that the zone of stasis can be saved by
the administration of recombinant tissue-type plasminogen activator (r-tPA).Its main
effect is on vessels coagulated by burn injury. We suggested that vessel patency might be
more important than other mediators released by burn injury. If this is so, the delay
phenomenon should completely save the zone of stasis in burns. Also, if the
pathophysiologies of the ischaemia zone in flaps and the zone of stasis in burns are the
same, there is no need to use the same material in research on the two zones. The present
study was designed to study the fate of the zone of stasis in delayed flaps that are
responding to ischaemia.
Material and method
The study group consisted
of 25 New Zealand white rabbits (weight 3.0 ± 0.5 kg). The animals were anaesthetized by
intramuscular injection of ketamine hydrochloride (1 mg/kg). All the back skin was shaved
with clippers. Five of the rabbits were used for a standard flap design - the midline of
the back was marked and a dorsal skin flap measuring 4 x 5 cin was elevated laterally to
the midline with a caudal pedicle. A silicone sheet 0.5 mm thick the same size as the flap
was placed beneath the elevated flap, which was then fixed to the recipient bed by means
of silk skin sutures. Three days later the sutures were removed and the delayed flap was
completed to the size of 4 x 10 cm. by adding the caudal portion to the flap under
anaesthesia. The immediate flap of 4 x 10 cin was also elevated on the left side,
identically to that on the right. The flaps were sutured to their recipient beds. One week
later, the distal necrotic areas of the flaps were drawn on mm-square sheets, and the
percentages of the distal necrotic areas were calculated.
The second experimental group consisted of ten rabbits. After the above-described
preparation of the back skin, the right-hand delay flap was elevated in two stages. After
the first week of delay, the blood flow in the delayed flap and in normal skin was
measured by laser Doppler (Laserflo BPM2, Vasemedics, USA) with a skin probe (Vasamedics,
cat. N°P440). The measurements were performed in three different points of the distal and
proximal flap portions, holding the probe in position for 1 min. The average blood flow in
each part was calculated as a perfusion unit (PU) (PU = mI LD/min/100 g tissue). A brass
probe with a leg (0.5 x 1 em) was immersed in boiling water until thermal equilibrium was
achieved. The probe was then applied for 20 see to the delay flap 2 em from the distal and
media] edges of the flap, without any pressure being applied to it. After 5 min, the
heated probe was again applied for 20 sec to the flap 0.5 cm laterally to the previously
burned area. Another burn row was created in the same fashion 0.5 em proximally to the
first burn rows. Four burn rows with a cross-shaped interspace area between them were thus
formed (Fig. 1). The same pattern of burn injury was created on the skin on the
left side, without any flap. Blood flow measurements were performed in the interspaces 24
h and 7 days post-burn. At each testing time, five rabbits were prepared for nuclear
imaging and autoradiography, which are described in the following sections. Tissue
specimens including the burned areas were excised. The donor skin sites were repaired
primarily and the rabbits were allowed to live.
In the third experimental group (N°= 5), the delay flap on the right side was elevated in
two stages (N°= 10). One week later, an acute flap the same size was elevated on the left
side (Fig. 2). The same burn patterns were created in both flaps, and cross-shaped
interspaces were thus created. The burned flaps were tested using the same parameters 24 h
and 7 days post-burn (five at each testing time).
Nuclear imaging and autoradiography. At testing times, the rabbits were injected
with 6 mCi of technetium-99m methoxyisobutylisonitrile (99Tcm-MIBI) via the femoral veins
under pentobarbital anaesthesia. The specimen, including healthy skin measuring 2 cm
around the burned area, was removed after 30 min. The donor areas were closed primarily.
The specimen was placed on a translucent film layer (thickness 0.2 min), which was used as
a carrier in order to prevent distortion of the specimen and spreading of the radioactive
agent by extravasated fluids. It was immediately put under a gamma camera (Starcam 400,
General Electrics, USA) and images were obtained with a pinhole collimator for 15 min with
a 256 x 256 matrix. The uptake of the radioactive agent was expressed as a percentage of
the radioactivity taken up in the interspaces compared with that taken up in the burned
areas. After completion of nuclear iinaging, the specimen was used for direct
autoradiography. It was put on a film (Hypertihn-MP, Amersham, UK) in a dark room with
close contact and both were then placed in an autoradiography cassette fflypercassate,
Amersham,UK). After 24 h of exposure at -70 'C, the film was developed using an automatic
processor (Cronex T-G Processor, Dupont, USA).
The necrotic/viable tissue borders obtained from the autoradiographies were marked and the
total necrotic area was counted with the aid of a semitransparent sheet with Imul 2 grids.
Analysis. The blood flow measurements, radioactive agent uptake percentages, and
necrotic areas obtained from the autoradiographies were expressed in terms of mean
standard deviation. Significance was assigned at p < 0.05 and the values were
statistically compared using the paired twotailed Student's t test.
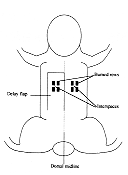 |
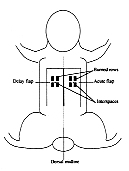 |
| Fig. 1 - Schematic drawing of delay flap and burn rows
applied in phase 2 of the study |
Fig. 2 - Schematic drawing of delay and acute flaps and
burn rows in phase 3 |
|
Results
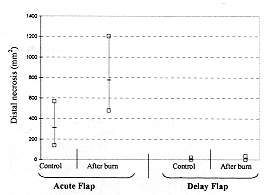
In the first group, the average necrotic area of the acute flaps was 310.4 mm2,
while nearly all the delayed flaps survived with an average necrotic area of 4 mm2.Burn
rows were then created 2 cin proximally from the distal edges of the flaps in groups 2 and
3. When the delay flap was compared with normal skin in group 2, the necrotic changes
between the burn rows began to be seen at the 24th h and were comparable with the process
on day 7 post-burn (Figs. 3, 4).
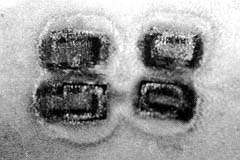 |
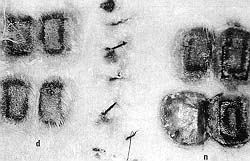 |
| Fig. 3 - The appearance of interspaces in the delay flap
24 h postburn. Note the progressive necrosis between the rows. |
Fig. 4 - Comparative appearance of necrosis in delay flap
(d) and normal skin (n). |
|
No significant
differences were found in the laser Doppler flowmetry measurements and gamma camera counts
at test times. When compared with healthy skin, nuclear imaging revealed that the uptakes
in the burned areas ranged between 20 and 40% (Fig. 5).
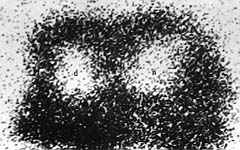 |
| Fig.
5 - Nuclear imaging of the specimen shown in Fig. 4. |
|
The
necrotic areas, which were calculated from the autoradiographies in group 2, were found to
be 316 ± 62 mat' in normal skin and 274 ± 45 mm2 in delay flaps on day 7
post-burn (Figs. 6, 7).
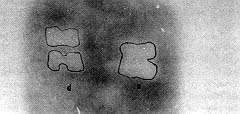 |
 |
| Fig.
6 - Autoradiography of the delay flap (d) versus normal skin (n). Note the marked
distinct borders of the necrotic areas. |
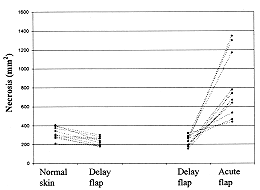
Fig
.7 - Grapfic representation of necrotic area (mm2)obtained from
autoradiographies.Each line represent one rabbit. |
|
When the
delay flaps were compared with the acute flaps in group 3, a significant decrease was
observed in the distal circulation of the acute flap, and a rate of 4.5 ± 2.2 PU was
found in the delay flaps on day 7 post-burn. Distal necrosis in the acute flaps increased
significantly to 774 ± 138 mm2 at this stage, while it was 12 ± 4 mm2
in the delay flaps (Figs. 8, 9).
 |
 |
| Fig .8 - Appearance of necrosis in the delay flap versus
the acute flap on day 7 post-burn |
Fig. 9 - Graphic representation of distal flap necrosis
in phase 1 and 3 studies (acute versus delay flaps). |
|
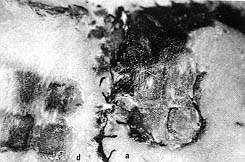
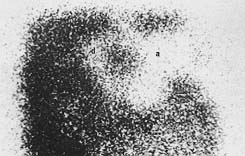
Nuclear
imaging revealed 0-10% uptakes of interspaces in the acute flaps (Fig. 10). The
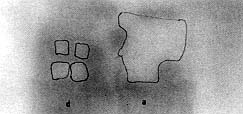
area of necrotic change in the burned zones was 288 ± 54 mm2 in the delay
flaps and 680 ± 41 mm2 in the acute flaps (p < 0.023)
(Figs. 7, 11).
 |
 |
| Fig.10 - Nuclear imaging of delay (d) versus acute (a)
flaps. Note the distal and extensive decreased uptakes in the acute flap. |
Fig. 11 - Autoradiography of a specimen in the phase 3
study. Note the marked distinct necrosis borders and extensive necrosis of the acute flap
(a). |
|
Discussion
The present study
describes a dorsal skin flap model in rabbits. This model was used because its width is
sufficient for the elevation of two flaps. It is advisable to use two flaps with burn rows
in the same animal, since the metabolic conditions in different rabbits may affect flap
survival. It was not possible to find any axial vessels in the pedicles of our flaps and
all the vessels were positioned perpendicularly to the long axis of the flaps, as shown in
Fig. 12.
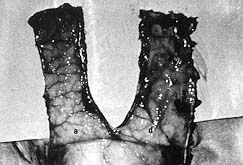 |
| Fig.
12 - Appearance of undersurface of acute (a) and delay (d) flaps. Note the
direction of vascular territories clearly seen in the acute flap. These territories could
not be seen so easily under the delay flap because the silicone sheet placed under the
delay flap caused thickening of the flap. |
|
We
hypothesized that this type of vessel territory might be beneficial for the opening of
anastornotic and choked vessels in delayed flaps where blood flow has been maintained.
Morris and Taylor showed that the delay phenomenon would have its maximum effect 48-72 h
after elevation of the rabbit skin flap. On the basis of these findings, we planned to
elevate the delay flap in two Stages. First, half the long axis of the flap was raised and
then, three days later, the size of the delayed flap was increased to 4 x 10 cm. A
silicone sheet was placed between the delayed flap and its donor area in order to prevent
the skin graft effect and to achieve a continuous response to the delay, as shown by
Hammond` in the rat with the McFarlane flap model. The present study showed a nearly 100%
survival rate in the delayed dorsal flap in the rabbit and an average rate of 86% in the
acute flap.
Regas and Ehrlich 14 were the first to describe the "comb burn model" for the
evaluation of the zone of stasis in burns. We have made some slight modifications to this
model. Each row was burned at different times because we suspected the presence of burn
injury in the interspace area between the two legs of the burning device owing to heat
convection. In the present study, the interspace areas were largely protected from direct
burn injury. A crossshaped interspace area was used. It may be hypothesized that if a
tissue responding to ischaernia is spared from burn injury, the direction is less
important. Also, the mediators released from the burn rows would be more effective in this
cross-shaped pool. We observed that the part of the delay flap distal to the burn can be
saved even if the circulation in this area is impaired by burns.
Laser Doppler blood flowmetry and gamma camera images established the circulation status
of each area. Our results showed a slightly increased circulation at the base of the
flaps. The circulation was impaired in the distal parts of the acute flaps and was nearly
normal in the delay flaps, compared with normal skin. These techniques did not however
define the exact borders of the necrotic areas. Autoradiography gives objective data for
the calculation of survival rates. This simple technique is the method of choice when
spatial resolution is more important than absolute sensitivity.` Autoradiography produces
quantitative images in which the absorbency of the film image is directly proportional to
the amount of radioactivity. If there had been access to a digital autoradiography system,
the results would have been quantitated.
With regard to the flaps, the burn injury increased the distal necrotic area of the acute
flaps in this study. This can be attributed to a partial decrease in blood flow to. the
already ischaemic distal parL Conversely, the burn injury did not further increase the
necrofic part of the delayed flap. This may be explained by the rich, dilated anastomotic
and choked vessels in delayed flaps, a finding that was confirmed histologically.These
vessels probably saved the part distal to the burn rows. The difference between the
necrotic areas of the delayed and the acute flaps suggested two different
pathophysiological mechanisms that accentuated each other in the acute flaps. Burn oedema
and some mediators released from the burned areas are not included in the pathophysiology
of ischaemic necrosis. The flap survival rates may suggest that these factors do not
affect tissues responding to ischaemia or that they are cleansed from the environment by
the blood circulation in delayed flaps.
When the survival rates of the interspaces between burned rows on delayed flap and of
normal skin obtained from the autoradiographies were compared, a slight, statistically
non-significant difference was found. The delay phenomenon was not capable of saving more
interspace area than normal skin. In other words, anatomically dilated choked vessels in
delay flaps were unable to save the interspaces. This may be due to additional
pathophysiological mechanisms acting on tissues responding to ischaemia. There may be
constriction and/or coagulation of these choked vessels or some necrofizing mediators may
have been released from the burned areas, e.g. thromboxane A2. These vasoactive agents
mostly act on vessels by vasoconstriction and platelet adherence. In our recent study, we
showed the effect of a potent fibrinolytic agent (recombinant tissue-type plasminogen
activator) on the saving of the zone of stasis in rats.' We suggested that maintenance of
vessel patency could save the zone of stasis. In a recent study, Tan et aU showed that
superoxide dismutase did not prevent post-burn dermal necrosis, although they found
increased levels of malonyl dialdehyde in burned skin tissue. Nevertheless, the effect of
the delay phenomenon was apparent when compared with intersPaces in acute flaps where all
the interspaces became necrotic. This finding also suggested that an additional mechanism
involved in burns leading to ischaemia resulted in total necrosis of the zone of stasis.
As a result, the term "progressive burn ischaemia", used to describe the
pathophysiological events in the zone of stasis, seems appropriate, where the terms
"progressive" and "burn" are used together with ischaemia because the
mechanisms involved in burns are apparently different from those involved in flap
ischaemia.In conclusion, it seems that the mechanism involved in saving ischaemic tissues
does not significantly save the zone of stasis in burns. There would appear to be
different pathophysiological events or mediators in burns and ischaernia that accentuate
each other's effects. Research on these challenging entities should be undertaken
separately, using some recently discovered mediators in each condition.
RESUME. Pour étudier le destin de la peau qui répond à
l'ischémie après les brûlures expérimentales, les Auteurs ont utilisé 25 lapins
blancs de la Nouvelle-Zélande (poids moyen 3,0-3,5 kg), divisés dans trois groupes. Dans
le premier groupe, les Auteurs ont élevé des lambeaux dorsaux retardés (4 x 10 cm)
munis de pédicule caudal (n' 5) et suturés aux sites donneurs. Au 7ème jour après
l'élévation, des lambeaux aigus dorsaux ont été élevés contre-latéralement aux
lambeaux retardés. Après une semaine, la superficie des zones nécrotiques a été
calculée. Dans le deuxième groupe, les lambeaux retardés ont été élevés et des
rangs de brûlures ont été créés dans la moitié distale du lambeau (n' 10). Une sonde
(0,5 cm) a été réchauffée dans de l'eau bouillante et appliquée quatre fois sur la
peau pour 20 sec afin de créer un espace interspatial à tonne de croix (largeur 0,5 cm).
Le même dessin de brûlure a été créé sur la peau normale près du lambeau. Dans le
troisième groupe, les lambeaux retardés et les lambeaux aigus ont été élevés dans la
même façon et le même dessin de brûlures a été créé dans les moitiés distales des
lambeaux. Les Auteurs ont effectué des tests, y compris la débitmétrie au laser doppler
et l'autoradiographie le premier jour et le 7ème jour après la brûlure. Dans le premier
groupe, l'aire moyenne nécrotique des lambeaux aigus était de 310,4 mm2, et
tous les lambeaux retardés ont survécu. Les rangs de brûlures ont été créés à 2 cm
proximalement aux bords distaux des lambeaux dans les groupes 2 et 3. Dans le 3ème groupe
la nécrose distale des lambeaux aigus augmentait en manière significative à 774 ± 138
mm2 au 7ème jour. Le mesurage laser doppler de la débitmétrie a révélé
une circulation augmentée distale dans les lambeaux aigus, et une unité de perfusion de
4,5 ± 2,2 dans les lambeaux retardés au 7ème jour après la brûlure. La quantité des
changements nécrotiques dans les zones brûlées du groupe 2, calculée sur la base des
autoradiographies, a été évaluée comme 316 ± 62 mm2 dans la peau normale
et 247 ± 45 mm2 dans les lambeaux retardés au 7ème jour. Dans le groupe 3
les résultats étaient 288 ± 54 mm2 dans les lambeaux retardés et 680 ± 41
mm2dans les lambeaux aigus. En conclusion les Auteurs considèrent que la
mécanisme qui préserve les tissus ischémiques ne sauve pas en manière significative la
zone de la stase dans les brûlures.
BIBLIOGRAPHY
Robson M.C., Del Baccaro E.J.,
Heggers LP.: The effect of prostaglandins on the dermal microcirculation after burning,
and the inhibition of the effect by specific pharmacological agents. Plast. Reconstr.
Surg., 63: 781-8, 1979.
Choi M., Rabb H., Amaut M.A. et al.:
Preventing the infiltration of leucocytes by monoclonal antibody blocks the development of
progressive ischaemia in rat burns. Plast. Reconstr. Surg., 96: 117787, 1995.
Arturson G.: Pathophysiology of the burn wound and pharmacological treaurnent. The Rudi
Hermans Lecture, 1995. Burns, 22: 255-74, 1996.
Williams W.G., Phillips L.G.: Pathophysiology of the burn wound. In: "Total Burn
Care" (Ist edition), Herridon D.N. (ed.), W.B. Saunders, Philadelphia and London,
1996.
Battal M.N., Hata Y., Matsuka K. et al.: Reduction of progressive burn injury by using a
new nonselective endothelin-A and endothelin-B receptor antagonist, TAK-044: An
experimental study in rats. Plast. Reconstr. Surg., 99: 1610-9, 1997.
Cetinkale Q., Demir M., Sayman H.B. et al.: Effects of allopurinol, ibuprofen and
cyclosporin A on local microcirculatory disturbances due to burn injuries. Burns, 23:
43-9, 1997.
Tan Q, Ma W-X., Wang L., Chen H.-R.: Can superoxide dismutase prevent post-burn dermal
ischaemia? Burns, 23: 228-31, 1997.
Isik S.,Sahin U., Ingan S. et al.: Saving the zone of stasis in burns with recombinant
tissue-type plasminogen activator (r-tPA): An experimental study in rats. Burns, 24:
217-23, 1998.
Daniel R.K., Kerrigan C.L.: Principles and physiology of skin flap surgery. In:
"Plastic Surgery", vol. 1 (Ist edition), McCarthy J.G. (ed.), W.B. Saunders,
Philadelphia and London, 1990.
Callegari P.R., Taylor G.I., Caddy C.M., Minabe T.: An anatomic, review of delay
phenomenon: 1. Experimental studies. Plast. Reconstr. Surg., 89: 397-407, 1992.
Taylor G.I., Corlett R.J., Caddy C.M., Zelt R.G.: An anatomic review of delay
phenomenon: 11. Clinical applications. Plast. Reconstr. Surg., 89: 408-16, 1992.
Morris S.F., Taylor G1: The time sequence of the delay phenomenon: When is a surgical
delay effective? An experimental study. Plast. Reconstr. Surg., 95: 526-33, 1995.
Hammond D.C., Brooksher R.D., Mann R.J., Beernink J.H.: The dorsal skin-flap model in
the rat: Factors influencing survival. Plast. Reconstr. Surg., 91: 316-21, 1993.
Regas F.C., Elirlich H.P.: Elucidating the vascular response to burns with a new rat
model. J. Trauma, 32: 557-63, 1992.
Boyd G.A.: "Autoradiography in Biology and Medicine", Academic, New York,
1955.
Address correspondence to:
Yrd. Doc. Dr. Selcuk lsik, GATA Plastik ve RekonstrUktif Cer. AD, Etlik 06018, Ankara,
Turkey.
This paper was received on 11 February 2000. |
|











