Annals oj'the MBC - vol. 2 - n' 3 -
September 1989
EPIDERMALIZATION OF
AN ARTIFICIAL DERMIS MADE OF COLLAGEN
Vescovali C*, Damour 0.**, Shahabedin L.**, David M.F.*,
Dantzer E.*, Marichy J.**, Collombel C.**, Echinard C*
* Laboratoire de Recherches chirurgicales, Unité de
Recherche sur les substituts cutanés et Hôpital de la Conception, Centre des Brûlés,
Marseille, France
** Laboratoire Biochimie C., Hôpital Edouard Herriot et Centre des Brûlés, Lyon,
France (Echinard C.) Laboratoire de Recherches Chirurgicales, Faculté de médecine,
Marseille, France
SUMMARY. Since 1987 we
have been developing a cultured epidermis, using H. Green's technique. This epithelium was
intended to cover skin losses such as extensive burns or excisions of giant naevi. Skin
was harvested from the patient when he was admitted; 1.5 X 106 keratinocytes were cultured
with 2 x 106 3T3 fibroblasts. After 10 days, a subculture was performed. On day 21, the
sheets of cells were grafted onto patients or animals. Results with this single technique
were not very satisfactory: the quality of the skin was poor and contractures often
appeared within 2 months. A fibrotic scar was formed under the epithelium. For this reason
we also developed an artificial dermis made of human collagen, chitosan and
glycosaminoglycans. The epidermal ization of this dermis was then undertaken. This was
first performed in vivo in rats and nude mice. These works are still in progress. In
vitro epithelialization was also done using Green's or Boyee's technique on artificial
dermis prior to grafting. Recent data about these experimental studies will be reported.
Since 1987, we have been performing
epidermal cell cultures according to Green's technique (6). This allows us to obtain,
within 21 days, an epidermal sheet grown from burned patient keratinocytes. Harvesting of
the cells is done 24 hours after admission of the patient to hospital: a 3 CM2 full
thickness skin piece is taken under sterile conditions.
Epidermal cells (EQ are separated from dermis (i.e. fibroblasts) by trypsin EDTA.
EC are then grown on a layer of irradiated 3T3 mice fibroblasts that are used as feeder
cells: 2 x 106 3T3 together with 2 x 106 keratinocytes.
The culture medium, containing DMEM, HAM, FCS, hydrocortisone, insulin, cholera-toxin,
glutamine, streptomycin and penicillin, is supplemented on day 3 with epidermal growth
factor.
After 10 to 12 days cells are confluent. They are dissociated by trypsinization and
regrown on another 3T3 feeder layer in order to perform a larger subculture. Within the
next 10 days, EC are forming a nice sheet (Fig. 1) which can be detached from the bottom
of the flask after incubation with dispase, and without dissociation of the cultured
cells. EC sheets are then attached and clipped on gauzes and can be transferred to
patients. Such an epithelium can be used for covering extensive burn patients or children
with giant naevi.
However, though this technique is biologically excellent, it seems to give unsatisfactory
clinical results. The quality of the reconstructed skin is poor, and shrinkage and
contraction often occur. This is most frequently due to the granulation tissue of the
underlying wound bed being infected and containing retracting myofibroblasts.
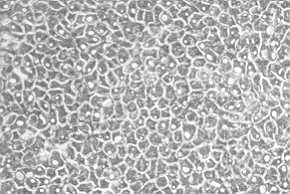
|
Fig. 1: Layer of
cultured keratinocytes after sub-culture (Green's technique). Optical microscopy. |
|
For this reason we have been
simultaneously working on the fabrication of an artificial dermis, partly according to
Burke's (2,7) theory, in order to obtain a hybrid artificial full thickness skin,
comparable to normal skin with its two layers: epidermis and dermis.
This artificial dermis is constituted of an extracellular matrix made of human collagen
(I, III, IV), chitosan and glycosaminoglycans. These last two components form an ionic
network and give the matrix its specific three-dimensional structure. Our previous works,
reported last year, showed no toxicity towards cells, good elasticity, and good tolerance
to collagenase (Mediterranean Burns Club Ist meeting 1987).
In vivo biocompatibility was done in the Sprague Dawley rat, showing that the
grafted artificial dermis is progressively recolonized by cells and vessels (3).
On day 2, the dermis is still separated from the receiving bed by oedema and exudate.
On day 20, the dermis has become a real neodermis with nicely orientated fibroblasts, very
different from granulation tissue with its anarchic colonization and vertical
vascuiarization (4,5).
We then endeavoured to epidermalize this artificial dermis. First studies were carried out
in vivo in Sprague Dawley male rats and nude mice. When the sheets of artificial
dermis and epidermis (grown according to Green's technique) were applied together at the
same time on a full thickness wound of the back; results were not very satisfactory.
Usually the epidermis became necrotic before the dermis could be completely colonized and
revascularized. When the epidermis was grafted a week after the dermis was put on the back
of the animal, we could obtain a living full thickness skin by day 20. However, due to the
problems of dressings in animals, results were not as good as expected.
The next step was the graft of artificial dermis in burn patients.
A 30-year-old lady sustained a 45% full thickness flame burn. She was very deeply burned
on the chest and abdomen. An early excision down to the fascia was performed on day 3
post-burn and artificial dermis was grafted at the same time (Fig. 2), a piece of healthy
skin was harvested and an epidermal cell culture was begun. The aspect of the wound on day
5 after excision and grafting of the dermis was quite good.
The histological study carried out at that time showed a good recolonization of the
artificial dermis. This phenomenon seems to happen faster in human beings than in animals.
On day 21, the cultured epidermis was grafted on revascularized dermis: nearly the whole
abdomen was grafted this way. A mesh graft was put on the breast.
Results were again not very satisfactory. 75% of the cultured epidermis was not adhering
to the revascularized artificial dermis. However, in some places where the epidermal graft
took, the histological study showed a beautiful aspect of reconstructed full thickness
skin. On day 5, the epidermis was not perfectly attached to the dermis; on day 20, the
dermal epidermal junction was complete.
These results led us to think that we could also fabricate a full thickness skin in
vitro. Two techniques of cell cultures were used: Green's technique, as previously
described, and Boyce's technique, using a definite medium without feeder cell layer (1).
With Green's technique 5 to 6 layers of keratinocytes can be seen on day 12 on the top of
a matrix in which there are no cells but a nice bundle of collagen. With Boyce's method,
the same kind of aspect can be seen on day 10 (Fig. 3). Works in progress tend now to
study the behaviour of such a full thickness skin prepared in vitro once it is
grafted to animals of Daniere and Coll.
When this type of composite graft (Artificial Dermis + non confluent cultured
keratinocytes) is put on the back of a nude mouse, after an 8-day culture, the whole graft
becomes recolonized by mesenchymal cells and revascularized. On day 15 post-graft we can
get a normal full skin (Fig. 4). This is the point we have reached. This kind of
artificial full thickness skin, if it works on man, is still consistent with the early
excision and immediate grafting concept, which can be done at the end of the first week
post-burn.
 |
 |
| Fig. 2: Excision of a
deep burn on the chest, and immediate coverage by artificial dermis. |
Fig. 3: In vitro, culture
of human keratinocytes on the extra-cellular matrix of dermis (day 10). |
|
Conclusions
Early surgical treatment of extensive
burns is related to two major conditions: rapidity of the procedure and quality of the
result.
Rapidity means early excision: this makes it possible to avoid metabolic disorders and
immunosuppression which lead to infection.
The quality of the result is due to immediate coverage of the wound in order to avoid
granulation tissue responsible for hypertrophic scars and contractures.
The isolated techniques of Green, Bell or Prunieras are excellent biological techniques,
but they need 3 weeks to be achieved and therefore do not allow early excisions. On the
other hand, the use of epidermis alone is not satisfactory.
Reconstruction of full thickness skin is like building a house: a good foundation is early
excision of the burn, the body of the house is the dermis. The roof is the epidermis.
Between them the beams are constituted by the dermal/epidermal junction.
This full thickness skin is a hybrid artificial skin: the biological part is the epidermis
made of living keratinocytes. The artificial part is the extra-cellular matrix that
becomes also a real living tissue once it is colonized by the patient's own fibroblasts.
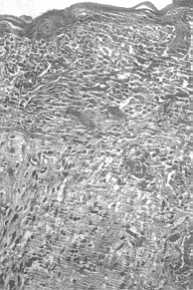
|
Fig. 4:
Extra-cellular matrix of dermis seeded with in vitro cultured keratinocytes and
grafted on the 8th day of culture. Aspect of the graft on day 15 in vivo. |
|
RÉSUMÉ. Un derme
artificiel français a été développé ces dernières années pour couvrir les grands
brûlés. Il est constitué de collagène humain, de glycosaminoglycannes et de chitosan.
Ce dernier constituant permet une rêticulation stable et non chimique entre les fibres de
cette matrice extracellulaire. Il a, en outre, une action sur l'immunité locale, sur
l'hémostase et peut être utilisé comme support de cultures cellulaires. Le derme
artificiel mis en place est complètemente réhabité et revascularisé en quelques jours
et devient un véritable tissu vivant dont les qualités sont identiques à un derme
normal. Nous présentons ici les tentatives d'épidermisation de cette matrice
extracellulaire, in vivo et in vitro, en un temps ou en deux temps de
manière à obtenir une peau bioartificielle totale utilisable en chirurgie plastique et
chez les grands brûlés.
BIBLIOGRAPHY
- Boyee C., Ham R.G.: Ca-regulated differentiation of
normal human epidermal keratinocytes in chemically defined clonal culture and free serial
culture. J. Invest. Dermatol., 9-2: 83-93, 1983.
- Burke J.F., Yannas I., Quiriby C., Bondoc C., Jung
W.K.: Successful use of a physiologically acceptable artificial skin in treatment of
extensive burn injury. Annals of Surgery, 194: 413-428, 1981.
- Echinard G, Dantzer E., Damour 0., Poinsignon F.,
Chabert B., Collombel C.: Use of artificial dermis for skin loss repair. 11 Trattamento
delle ferite, 85-87, Monduzzi Editor, 1987.
- Echinard C., Dantzer E., Poinsignon F. et al.: Mise
au point d'un derme acellulaire, un pas vers la peau artificielle totale. Ann. Chir.
Plast. Esth. 1988. In press.
- Echinard C., Damour 0., David M.F., Vescovali C. et
al.: In vivo and in vitro studies of a hybrid artificial skin. In:
"Surgical Updating 1988". International College of Surgeons, Montorsi Publ. Vol.
111, Monduzzi Editore. Bologna, Italia.
- Green H., Kehinde 0., Thomas J.: Growth of cultured
human epidermal cells into multiple epithelia suitable for grafting. Proceedings of the
National Academy of Sciences, 76: 5665-5666, 1979.
- Yannas I., Burke L, Orgill D., Skrabut E.: Worend
tissue can utilise a polymeric template to synthesize a functional extension of skin.
Science, 215: 174-176, 1982.
|



